Abstract
Aim:
Pseudoneurological complaints (PNCs) are highly prevalent among the general population. Coronavirus disease 2019 (COVID-19) adversely influences such complaints in individuals who recovered from COVID-19. This study determined the prevalence and identified the predictors of PNCs among individuals who had previously experienced COVID-19 and their healthy counterparts.
Methods:
This case-control study analyzed the data of 878 Bangladeshi adults (439 patients). Laboratory-confirmed COVID-19 individuals were considered cases, and the controls were those who never tested positive for COVID-19. The controls were matched with cases’ sex and age. The seven-item pseudoneurological sub-scale of the subjective health complaints scale produced by Eriksen et al. evaluated PNCs. The descriptive analysis estimated the prevalence of PNCs among the subgroups, whereas multiple logistic regression models were used to determine the predictors of PNCs.
Results:
Overall, the prevalence of PNCs was 40%; however, patients who recovered from COVID-19 reported a PNC rate of 67.4%. The regression analysis identified COVID-19 as a robust independent predictor of PNCs. Furthermore, occupation, monthly household income, current living location, hypertension, and recovery period from acute COVID-19 were independently associated with PNCs.
Conclusions:
This study revealed a significant association between COVID-19 and PNCs. The results of this study will be helpful when discussing, planning, and implementing strategies to alleviate the overburden of PNCs among COVID-19 survivors.
Keywords
Anxiety, COVID-19, depression, fear, economic crisis, pandemic, pseudoneurology, subjective health complaintsIntroduction
Pseudoneurological complaints (PNCs) are part of everyday subjective health complaints without significant pathological causes; however, they can cause major problems that may lead to serious illness [1]. Sleeping problems, palpitation (throbbing heart rate), heat flashes, weariness, dizziness, anxiety, and depression are considered major PNCs [2]. Previous studies have reported a high prevalence of PNCs among apparently healthy individuals [3]. However, earlier analyses have suggested that neurological problems are significantly prevalent among patients with acute coronavirus disease 2019 (COVID-19) [4]. Additionally, a symptom that appears in acute cases persists as the post-acute sequela of severe acute respiratory syndrome coronavirus 2 infections [5].
Since 2019, COVID-19 has infected more than 8% of the global population, and its prevalence has been increasing as the pandemic was unprecedently prolonged [6, 7]. The overburden of COVID-19 survivorship has been immense. Specifically, the heterogeneous manifestation of COVID-19 has affected the health of patients with COVID-19 and those without COVID-19. Pandemic-related fear, grief, loneliness, roaming restriction, and economic stressors negatively predict the neuropsychological health of the general population [8–10]. Most COVID-19 survivors have reported post-acute COVID-19 sign symptoms, including fatigue and exhaustion, dyspnea, coughing, headache, taste and smell alterations, and cognitive or psychological health dysfunction, namely anxiety and depression [11–13].
A few studies have examined the prevalence of neurological complaints among patients with long-term COVID-19. However, most of these studies have been conducted in high-income settings and among hospital patients [14]. COVID-19 has spread and affected health differently according to race and country; death and recovery rates are also unmatched in different locations [15]. Thus, it can be hypothesized that COVID-19 symptoms are unusual among patients who have recovered from COVID-19 in Bangladesh. To the best of our knowledge, the association between COVID-19 and PNCs has not been examined in the Indian subcontinental population. The current study examined the association between COVID-19 and a broad spectrum of pseudoneurological signs in patients with post-COVID-19 conditions and healthy individuals.
Materials and methods
Settings and respondents
This case-control study included 878 Bangladeshi adults (cases = 439). The cases were laboratory-confirmed patients who previously experienced COVID-19. The controls comprised individuals without COVID-19 who were matched with cases’ sex and age [16].
Sample size determination
Laboratory-tested 2.0 million COVID-19-positive cases across Bangladesh were considered the population [7]. A 95% confidence level, a 50% response distribution, and a margin with a 5% error were used to ascertain 385 respondents for case data [17].
Inclusion and exclusion criteria
Participants aged > 18 years residing in Bangladesh were included in this study. Patients with acute COVID-19, who were pregnant, and who were unreliable and unpredictable individuals (i.e., participants diagnosed with a severe psychological disorder, bedridden individuals, participants with severe chronic illnesses, particularly rheumatoid and gouty arthritis, cerebrovascular accident, or malignancy) were excluded from this study.
Data source and collection
Six previously trained expert researchers were engaged in the data collection process. Six hundred laboratory-tested post-acute COVID-19-positive individuals were collected from 10 conveniently selected government-affiliated COVID-19 testing centers in Bangladesh. After considering the inclusion and exclusion criteria, 450 participants were interviewed. The aims of this study were explained, documented, and scripted. Written informed consent was obtained from the expected participants to collect, analyze, and publish the data before the interview started. Finally, the participants were interviewed in person using a paper-based semi-structured questionnaire at their residences or occupational places. This study included 439 eligible data points for the “case” group.
Subsequently, data from 439 non-COVID-19 participants were collected. “Control” participants were selected from the case’s suitable family members, neighborhood, or office colleagues.
Therefore, 878 data points were collected between February 24 and April 7, 2022. Optimal confidentiality was maintained throughout the data collection process. Data were recorded anonymously and stored in a password-encrypted computer in an unidentifiable manner for analysis.
Questionnaire
Sociodemographic characteristics including age, biological sex, marital information, education, occupational history, and domestic monthly earnings in Bangladeshi taka (BDT) (1 US dollar = approximately 100 BDT), and present residence were included in the first part of the questionnaire. Phase two of the questionnaire assessed the respondents’ chronic comorbidities, particularly bronchial asthma, hypertension, diabetes mellitus, and renal disease, tobacco use history, and regular physical exercise habits. Dichotomous options (yes or no) were used to answer these questions.
For the case data, the participants provided details about their COVID-19. Symptom severity (very severe, severe, moderate, and mild), and the treatment amenities patients had used (hospital’s intensive care unit, hospital’s general ward, or home) were reported. The recovery duration from the acute COVID-19 vaccination status was also documented for this cohort.
The final part of the questionnaire assessed the pseudoneurological health complaints of the last 30 days using the pseudoneurology sub-scale of Eriksen et al.’s [18] Subjective Health Complaints scale. The PNCs included seven components: palpitation, heat flashes, sleeping problems, fatigue, dizziness, anxiety, and depression. The severity of PNCs was evaluated on a scale of four points (0–3: 0, none; 1, some; 2, much; and 3, severe). These complaints were additionally enumerated based on the number of days in the past month. Severity was multiplied by duration to obtain a complete score ranging from 0 to 90, signifying the degree of illness [18]. For this study, respondents who complained of at least some problems for 3 days (1 × 3 = 3) in the last month and scored ≥ 3 were considered to have PNCs.
Ethical consideration
The ‘Ethical Review Committee (ERC) of Uttara Adhunik Medical College’ cleared the ethical issue for this study (Approval number: UAMC/ERC/Recommend- 11/2021). Furthermore, prospective registration for this case-control study was received from the World Health Organization-endorsed Clinical Trial Registry, India (CTRI/2022/02/040449, registered on February 2, 2022). Formal written informed consent was obtained from all the participants before data collection to collect, analyze, and publish their data. The Strengthening the Reporting of Cohort, Cross-Sectional and Case-Control Studies in Surgery guideline [19] was strictly followed throughout the study.
Participants and public involvement
The respondents and the public were not engaged in our study design, conduct, reporting, and dissemination plans. The study’s endeavors and objectives were elucidated, and assurance of anonymity was granted before receiving written informed consent from the participants.
Analysis of data
The Statistical Package for the Social Sciences (version 28.0; IBM Corp., USA) was used to complete the data analyses. To compute the prevalence, the four responses to the PNC questions were dichotomized according to whether the participants were experiencing the symptoms (yes) or not (no). Chi-squared tests were used to compare categorical variables with and without PNCs. Multiple logistic regression models were used to calculate adjusted odds ratios (aORs), considering a confidence interval (CI) of 95% with PNCs as a measured variable and sociodemographic particularities and impersonal COVID-19 illness-associated components as the predictor variables for PNCs. The two regression models included only the statistically significant variables in the descriptive analyses. The Hosmer–Lemeshow goodness-of-fit test tested the fitness of the model. P-values < 0.05 were considered statistically significant.
Results
General characteristics
This study analyzed 878 data (50.5% women) of patients with a mean age of 38.30 [standard deviation (SD) ± 12.77] years. There were 439 data (49.2% women) in the case group, and the mean age of this group was 38.33 (SD ± 12.53) years. Conversely, the control group comprised 51.7% of women, with a mean age of 38.28 (SD ± 13.01) years.
For all data, most of the participants were married (83.6%), graduates (34.1%), and jobholders (33.7%), had a monthly income of BDT > 45,000 (42.5%), belonged to the nuclear family (66.6%), and were urban dwellers (61.5%). Nonetheless, only 24.0%, 23.5%, 10.3%, and 16.4% of the participants reported hypertension, diabetes, kidney disease, and asthma, respectively. Additionally, approximately 19.8% and 38.8% of the respondents reported that they performed routine physical workouts and were tobacco addicts, respectively. The results are presented in Table 1.
Descriptive data of the entire respondents: sociodemographic and clinical variables and PNCs
| Variables | PNCs | Total (column %) | P-value | |
|---|---|---|---|---|
| No (row %) | Yes (row %) | |||
| Total respondents | 527 (60.0) | 351 (40.0) | 878 (100) | |
| Tested positive for COVID | < 0.001* | |||
| Yes | 143 (32.6) | 296 (67.4) | 439 (50.0%) | |
| No | 384 (87.5) | 55 (12.5) | 439 (50.0%) | |
| Biological sex | 0.401 | |||
| Female | 272 (51.6) | 171 (48.7) | 443 (50.5) | |
| Male | 255 (48.4) | 180 (51.3) | 435 (49.5) | |
| Age group (year) | 0.063 | |||
| 18–30 | 195 (62.1) | 119 (37.9) | 314 (35.8) | |
| 31–40 | 146 (53.9) | 125 (46.1) | 271 (30.9) | |
| 41–50 | 91 (59.1) | 63 (40.9) | 154 (17.5) | |
| 51–60 | 57 (67.9) | 27 (32.1) | 84 (9.6) | |
| > 60 | 38 (69.1) | 17 (30.9) | 55 (6.3) | |
| Marital status | 0.111 | |||
| Single | 95 (66.0) | 49 (34.0) | 144 (16.4) | |
| Married | 432 (58.9) | 302 (41.2) | 734 (83.6) | |
| Educational status | < 0.001* | |||
| ≤ High school | 140 (52.8) | 125 (47.2) | 265 (30.2) | |
| College education | 134 (61.5) | 84 (38.5) | 218 (24.8) | |
| Graduation | 193 (64.5) | 106 (35.5.0) | 299 (34.1) | |
| ≥ Post-graduation | 60 (62.5.) | 36 (37.5) | 96 (10.9) | |
| Employment status | < 0.001* | |||
| Jobholder | 180 (83.8) | 116 (39.2) | 296 (33.7) | |
| Businessman | 68 (51.1) | 65 (48.9) | 133 (15.1) | |
| Unemployed | 42 (59.2) | 29 (40.8) | 71 (8.1) | |
| Student | 46 (92.0) | 4 (8.0) | 50 (5.7) | |
| Home maker | 122 (70.1) | 99 (44.8) | 221 (25.2) | |
| Healthcare personnel | 69 (64.5) | 38 (35.5) | 107 (12.2) | |
| Monthly household income (BDT) | < 0.001* | |||
| < 15,000 | 58 (45.0) | 71 (55.0) | 129 (14.7) | |
| 15,000–30,000 | 70 (48.6) | 74 (51.4) | 144 (16.4) | |
| 31,000–45,000 | 155 (66.8) | 77 (33.2) | 232 (26.4) | |
| > 45,000 | 244 (65.4) | 129 (34.6) | 373 (42.5) | |
| Family category | 0.076 | |||
| Nuclear family | 339 (57.9) | 246 (42.1) | 585 (66.6) | |
| Joint family | 188 (64.2) | 105 (35.8) | 293 (33.4) | |
| Current residence | 0.007* | |||
| Rural | 115 (63.9) | 65 (36.1) | 180 (20.5) | |
| Urban | 303 (56.1) | 237 (43.9) | 540 (61.5) | |
| Semi-urban | 109 (69.0) | 49 (31.0) | 158 (18.0) | |
| Hypertension | 0.003* | |||
| No | 419 (62.8) | 248 (37.2) | 667 (76.0) | |
| Yes | 108 (51.2) | 103 (48.8) | 211 (24.0) | |
| Diabetes | 0.450 | |||
| No | 408 (60.7) | 264 (39.3) | 672 (76.5) | |
| Yes | 119 (57.8) | 87 (42.2) | 206 (23.5) | |
| Kidney disease | 0.499 | |||
| No | 470 (59.6) | 318 (40.4) | 788 (89.7) | |
| Yes | 57 (63.3) | 33 (36.7) | 90 (10.3) | |
| Asthma | 0.167 | |||
| No | 448 (61.0) | 286 (39.0) | 734 (83.6) | |
| Yes | 79 (54.9) | 65 (45.1) | 144 (16.4) | |
| Exercise habit | 0.071 | |||
| No | 433 (61.5) | 271 (38.5) | 704 (80.2) | |
| Yes | 94 (54.0) | 80 (46.0) | 174 (19.8) | |
| Current tobacco user | 0.001* | |||
| No | 345 (64.2) | 192 (35.8) | 537 (61.2) | |
| Yes | 182 (53.4) | 159 (46.6) | 341 (38.8) | |
*P-values signify 5% significance levels
In the case data (Table 2), a more significant part of the attendees was married (85.6%), graduated (33.9%), was jobholder (34.4%), earned BDT > 45,000 (45.1%), belonged to a nuclear family (67.9%), and resided in an urbanized area (68.6%). Nonetheless, approximately 27.3%, 26.7%, 14.4%, and 23.9% of the participants had hypertension, diabetes mellitus, renal illness, and asthmatic problems, respectively. However, approximately 24.4% and 44.9% of the respondents performed routine exercise and used tobacco, respectively. Similarly, a significant proportion of participants with post-acute sequelae COVID-19 recovered from the sickness > 180 days previously (36.7%), showed mild symptoms (49.9%), received treatment at home (65.1%), and received two doses of vaccines before apprehending this disease (53.0%).
Descriptive data of cases: sociodemographic and clinical factors related to COVID-19 and PNCs
| Variables | PNCs | Total (column %) | P-value | |
|---|---|---|---|---|
| No (row %) | Yes (row %) | |||
| Total respondents | 143 (32.6) | 296 (67.4) | 439 (100) | |
| Biological sex | 0.896 | |||
| Female | 71 (32.9) | 145 (67.1) | 216 (49.2) | |
| Male | 72 (32.3) | 151 (67.7) | 223 (50.8) | |
| Age group (year) | 0.135 | |||
| 18–30 | 48 (31.4) | 105 (68.6) | 153 (34.9) | |
| 31–40 | 40 (28.0) | 103 (72.0) | 143 (32.6) | |
| 41–50 | 25 (32.1) | 53 (67.9) | 78 (17.8) | |
| 51–60 | 18 (45.0) | 22 (55.0) | 40 (9.1) | |
| > 60 | 12 (48.0) | 13 (52.0) | 25 (5.7) | |
| Marital status | 0.091 | |||
| Single | 27 (42.9) | 36 (57.1) | 63 (14.4) | |
| Married | 116 (12.2) | 260 (87.8) | 376 (85.6) | |
| Educational status | 0.608 | |||
| ≤ High school | 37 (28.2) | 94 (71.8) | 131 (29.8) | |
| College education | 37 (34.3) | 71 (65.7) | 108 (24.6) | |
| Graduation | 50 (33.6) | 99 (66.4) | 149 (33.9) | |
| ≥ Post-graduation | 19 (37.3) | 32 (62.7) | 51 (11.6) | |
| Employment status | < 0.001* | |||
| Jobholder | 51 (33.8) | 100 (66.2) | 151 (34.4) | |
| Businessman | 17 (27.0) | 46 (73.0) | 63 (14.4) | |
| Unemployed | 12 (32.4) | 25 (67.6) | 37 (8.4) | |
| Student | 14 (82.4) | 3 (17.6) | 17 (3.9) | |
| Home maker | 31 (26.5) | 86 (73.5) | 117 (26.7) | |
| Healthcare personnel | 18 (33.3) | 36 (66.7) | 54 (12.3) | |
| Monthly household income (BDT) | < 0.001* | |||
| < 15,000 | 5 (8.9) | 51 (91.1) | 56 (12.8) | |
| 15,000–30,000 | 15 (20.3) | 59 (79.7) | 74 (16.9) | |
| 31,000–45,000 | 42 (37.8) | 69 (62.2) | 111 (25.3) | |
| > 45,000 | 81 (40.9) | 117 (59.1) | 198 (45.1) | |
| Family category | 0.269 | |||
| Nuclear family | 92 (30.9) | 206 (69.1) | 298 (67.9) | |
| Joint family | 51 (36.2) | 90 (63.8) | 141 (32.1) | |
| Current residence | < 0. 001* | |||
| Rural | 20 (33.3) | 40 (66.7) | 60 (13.7) | |
| Urban | 82 (27.2) | 219 (72.8) | 301 (68.6) | |
| Semi-urban | 41 (52.6) | 37 (47.4) | 78 (17.8) | |
| Hypertension | 0.350 | |||
| No | 108 (33.9) | 211 (66.1) | 319 (72.7) | |
| Yes | 35 (29.2) | 85 (70.8) | 120 (27.3) | |
| Diabetes | 0.175 | |||
| No | 99 (30.7) | 223 (69.3) | 322 (73.3) | |
| Yes | 44 (37.6) | 73 (62.4) | 117 (26.7) | |
| Kidney disease | < 0.001* | |||
| No | 108 (28.7) | 268 (71.3) | 376 (85.6) | |
| Yes | 35 (55.6) | 28 (44.4) | 63 (14.4) | |
| Asthma | 0.010* | |||
| No | 98 (29.3) | 236 (70.7) | 334 (76.1) | |
| Yes | 45 (42.9) | 60 (57.1) | 105 (23.9) | |
| Exercise habit | 0.786 | |||
| No | 107 (32.2) | 225 (67.8) | 332 (75.6) | |
| Yes | 36 (33.6) | 71 (66.4) | 107 (24.4) | |
| Current tobacco user | 0.865 | |||
| No | 78 (32.2) | 164 (67.8) | 242 (55.1) | |
| Yes | 65 (33.0) | 132 (67.0) | 197 (44.9) | |
| Recovering period after acute-COVID-19 | < 0.001* | |||
| < 30 days | 8 (66.7) | 4 (33.3) | 12 (2.7) | |
| 30–60 days | 16 (42.1) | 22 (57.9) | 38 (8.7) | |
| 61–90 days | 13 (11.8) | 97 (88.2) | 110 (25.1) | |
| 91–120 days | 15 (36.4) | 26 (63.6) | 41 (9.3) | |
| 121–150 days | 12 (36.4) | 21 (63.6) | 33 (7.5) | |
| 151–180 days | 15 (34.1) | 29 (65.9) | 44 (10.0) | |
| 180 + days | 64 (39.8) | 97 (60.2) | 161 (36.7) | |
| COVID-19 symptoms | 0.033* | |||
| Mild | 79 (36.1) | 140 (63.9) | 219 (49.9) | |
| Moderate | 23 (21.1) | 86 (78.9) | 109 (24.8) | |
| Severe | 17 (37.0) | 29 (63.0) | 46 (10.5) | |
| Very severe | 24 (36.9) | 41 (63.1) | 65 (14.8) | |
| Treatment facilities used | 0.182 | |||
| Home | 86 (30.1) | 200 (69.9) | 286 (65.1) | |
| Hospital general ward | 22 (32.8) | 45 (67.2) | 67 (15.3) | |
| Hospital intensive care | 35 (40.7) | 51 (59.3) | 86 (19.6) | |
| Dose of vaccine before COVID-19 | 0.010* | |||
| No vaccine | 53 (36.8) | 91 (63.2) | 143 (32.6) | |
| 1 Dose | 40 (41.2) | 57 (58.8) | 97 (22.1) | |
| 2 Doses | 44 (23.9) | 140 (76.1) | 184 (41.9) | |
| 3 Doses | 6 (42.9) | 8 (57.1) | 14 (3.2) | |
* P-values signify 5% significance levels
Descriptive analysis of the entire data
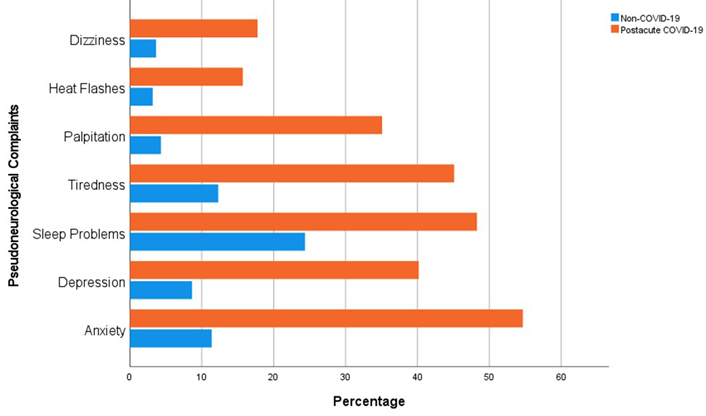
The prevalence of PNCs was 40.0% in all participants. Nonetheless, the incidence rate of PNCs was considerably higher in the case group than in the control group (67.4% vs. 12.5%, P ≤ 0.001). Closer analysis indicated that the prevalences of PNC components for the case and control groups were as follows: dizziness (17.8% vs. 3.6%, P ≤ 0.001), heat flashes (15.7% vs. 3.2%, P ≤ 0.001), palpitation (35.1% vs. 4.3%, P ≤ 0.001), tiredness (45.1% vs. 12.3%, P ≤ 0.001), sleep problems (48.3% vs. 24.37%, P ≤ 0.001), depression (40.2% vs. 8.7%, P ≤ 0.001), and anxiety (54.7% vs. 11.4%, P ≤ 0.001) (Figure 1). Additionally, participants with lower education (47.2%, P ≤ 0.001), with businesses (48.9%, P ≤ 0.001), who had lower gross monthly earnings (55.0%, P ≤ 0.001), who were city dwellers (43.9%, P = 0.007), who were diagnosed with hypertension (48.8%, P = 0.003), and who consumed tobacco (46.6%, P = 0.001) had significantly higher rate of PNCs (Table 1).
Descriptive analysis of case data
Evaluation of case data suggested a higher prevalence of PNCs among business people (73.0%, P ≤ 0.001), respondents with lower income (91.1%, P ≤ 0.001), city dwellers (72.8%, P ≤ 0.001), individuals who recovered from the illness 61–90 days previously (88.2%, P ≤ 0.001), who had moderate symptoms (78.9%, P = 0.033), and who received two doses of vaccine (76.1%, P = 0.010). In contrast, participants diagnosed with kidney disease (44.4%, P ≤ 0.001) and asthma (57.1%, P = 0.010) had lower PNC rates than those without kidney disease and asthma (Table 2).
Regression model of all data
Regression analysis 1 (Table 3) revealed the robust predictability of COVID-19 for PNCs (aOR, 21.97; 95% CI, 14.364–33.604) after adjusting for potential confounders. The highest odds of PNCs were also reported in participants with low income (aOR, 8.558; 95% CI, 4.287–17.087), who were city dwellers (aOR, 1.773; 95% CI, 1.092–2.879), and with hypertension (aOR, 1.903; 95% CI, 1.257–2.879).
Logistic regression analysis of all data: predictors of PNCs
| Factors | aOR | Standard error | 95% CI | P-value | |
|---|---|---|---|---|---|
| Category | |||||
| Case | 21.970 | 0.217 | 14.364 | 33.604 | < 0.001* |
| Control | Reference | ||||
| Education | |||||
| ≤ High school | 1.148 | 0.369 | 0.557 | 2.364 | 0.708 |
| Higher secondary education | 1.291 | 0.364 | 0.632 | 2.637 | 0.483 |
| Graduation | 1.058 | 0.318 | 0.567 | 1.975 | 0.860 |
| ≥ Post-graduation | Reference | ||||
| Employment status | |||||
| Jobholder | 0.988 | 0.308 | 0.541 | 1.807 | 0.970 |
| Businessman | 1.840 | 0.380 | 0.874 | 3.872 | 0.108 |
| Unemployed | 1.094 | 0.433 | 0.468 | 2.555 | 0.836 |
| Student | 0.103 | 0.664 | 0.028 | 0.380 | < 0.001* |
| Homemaker | 0.697 | 0.362 | 0.343 | 1.416 | 0.318 |
| Healthcare personnel | Reference | ||||
| Monthly household income (BDT) | |||||
| < 15,000 | 8.558 | 0.353 | 4.287 | 17.085 | < 0.001* |
| 15,000–30,000 | 3.052 | 0.302 | 1.687 | 5.521 | < 0.001* |
| 31,000–45,000 | 1.119 | 0.240 | 0.700 | 1.789 | 0.639 |
| > 45,000 | Reference | ||||
| Current residence | |||||
| Rural | 1.320 | 0.320 | 0.706 | 2.469 | 0.385 |
| Urban | 1.773 | 0.247 | 1.092 | 2.879 | 0.021* |
| Semi-urban | Reference | ||||
| Hypertension | |||||
| Yes | 1.903 | 0.247 | 1.257 | 2.879 | 0.002* |
| No | Reference | ||||
| Tobacco using | |||||
| Yes | 0.909 | 0.232 | 0.617 | 1.339 | 0.630 |
| No | Reference | ||||
* P-values signify 5% significance levels
Adjusted analysis of case data
Results of regression model 2 are shown in Table 4. In this case, significantly higher odds of PNCs were observed among participants with low income (aOR, 5.269; 95% CI, 1.631–17.023), who were city dwellers (aOR, 3.313; 95% CI, 1.754–5.592), and who recovered from the illness 30–60 days previously (aOR, 4.444; 95% CI, 1.641–12.033) and 121–150 days previously (aOR, 3.150; 95% CI, 1.053–9.419). Nonetheless, lower odds of PNCs were observed among students (aOR, 0.081; 95% CI, 0.018–0.361).
Logistic regression analysis of case data: predictors of PNCs
| Variables | aOR | Standard error | 95% CI | P-value | |
|---|---|---|---|---|---|
| Employment status | |||||
| Jobholder | 1.006 | 0.369 | 0.488 | 2.074 | 0.986 |
| Businessman | 1.361 | 0.473 | 0.539 | 3.435 | 0.515 |
| Unemployed | 1.829 | 0.550 | 0.623 | 5.374 | 0.272 |
| Student | 0.081 | 0.761 | 0.018 | 0.361 | 0.001* |
| Homemaker | 0.980 | 0.419 | 0.431 | 2.229 | 0.961 |
| Healthcare personnel | Reference | ||||
| Monthly household income (BDT) | |||||
| < 15,000 | 5.269 | 0.598 | 1.631 | 17.023 | 0.005* |
| 15,000–30,000 | 2.490 | 0.404 | 1.127 | 5.498 | 0.024* |
| 31,000–45,000 | 1.066 | 0.293 | 0.600 | 1.893 | 0.827 |
| > 45,000 | Reference | ||||
| Current residence | |||||
| Rural | 1.967 | 0.433 | 0.842 | 4.596 | 0.118 |
| Urban | 3.131 | 0.296 | 1.754 | 5.592 | < 0.001* |
| Semi-urban | Reference | ||||
| Kidney diseases | |||||
| No | 3.443 | 0.361 | 1.696 | 6.987 | 0.001* |
| Yes | Reference | ||||
| Asthma | |||||
| No | 1.417 | 0.284 | 0.812 | 2.475 | 0.220 |
| Yes | Reference | ||||
| Recovering period after acute-COVID-19 | |||||
| < 30 days | Reference | ||||
| 30–60 days | 4.444 | 0.508 | 1.641 | 12.033 | 0.003* |
| 61–90 days | 2.001 | 0.544 | 0.689 | 5.816 | 0.203 |
| 91–120 days | 1.915 | 0.581 | 0.613 | 5.981 | 0.264 |
| 121–150 days | 3.150 | 0.559 | 1.053 | 9.419 | 0.040* |
| 151–180 days | 1.721 | 0.460 | 0.698 | 4.239 | 0.238 |
| 180 + days | 0.407 | 0.775 | 0.089 | 1.861 | 0.247 |
| COVID-19 symptoms | |||||
| Mild | 0.594 | 0.374 | 0.285 | 1.234 | 0.163 |
| Moderate | 0.929 | 0.421 | 0.407 | 2.119 | 0.860 |
| Sever | 1.096 | 0.461 | 0.444 | 2.707 | 0.842 |
| Very severe | Reference | ||||
| Dose of vaccine before COVID-19 | |||||
| No vaccine | 1.766 | 0.684 | 0.462 | 6.747 | 0.406 |
| 1 Dose | 1.472 | 0.694 | 0.377 | 5.738 | 0.578 |
| 2 Doses | 1.523 | 0.670 | 0.410 | 5.658 | 0.530 |
| 3 Doses | Reference | ||||
* P-values signify 5% significance levels
Discussion
This comprehensive age- and sex-matched case-referent study revealed a high prevalence of PNCs among community dwellers in Bangladesh during the COVID-19 pandemic. A remarkably higher prevalence rate of PNCs was reported in individuals who previously experienced COVID-19 than in those who had never tested positive for COVID-19. Regression analysis confirmed the robust independent predictive ability of COVID-19 for PNCs. Occupation, monthly household income, current living location, and hypertension also predicted PNCs. In addition, the recovery period from acute COVID-19 was associated with PNCs.
Scarce information exists regarding subjective health complaints of the global population during the pandemic. A previous study reported that the prevalence of PNCs in Bangladesh was 26.6% [2]. This study reported a high prevalence rate of 40%. The prevalence rate was significantly higher (67.4%) in patients with post-acute COVID-19. In addition, the highest percentage of patients with post-acute COVID-19 complained of anxiety, followed by sleep problems, tiredness, depression, palpitation, dizziness, and heat flashes. In line with our outcomes, a methodological assessment and meta-analysis concluded that several patients with post-acute COVID-19 experienced anxiety, depression, fatigue, and cognitive impairment [13, 20].
Underprivileged populations with lower incomes are among the primary victims of the COVID-19 pandemic and the disease itself [21, 22]. Our current study also recommended that a significantly higher number of participants with household incomes of ≤ 15,000 BDT had complaints about PNCs. This heterogeneity may be due to the poor access of these individuals to healthcare facilities or the proper treatment of COVID-19 during or after acute illness. By contrast, this study revealed that city dwellers complained about PNCs at a significantly higher rate. People living in urban settings are more prone to depression, anxiety, and other neuropsychological health issues than those living in rural settings [23]. In line with other studies [24, 25], our analysis reported an unmatched prevalence rate of PNCs throughout the duration of the acute illness. Additional studies are warranted to understand the causal association between COVID-19 and PNCs.
This study revealed that the days that passed from the recovery from acute COVID-19 influenced the prevalence of PNCs. This study suggested that the symptoms were highly prevalent between one to two months of recovery from acute illness, and the prevalence reduced after two months and then increased after three months until five months. In line with the findings of current study, previous study results showed similar fluctuations in the prevalence of post-acute COVID-19 symptoms based on the duration of recovery from acute COVID-19 [26]. Nonetheless, this study suggested that the prevalence of PNCs was lower among the patients diagnosed with kidney and asthma. More investigations are warranted to understand the causal relationship between comorbidities and PNCs among patients with post-acute COVID-19.
Strengths and limitations
To the best of our knowledge, this is the first case-control study that compared PNCs among patients with post-acute COVID-19 and healthy individuals. Furthermore, this study added COVID-19-related data from a low-resourced country in the literature, which is scarce. However, this study has some limitations. First, this study measured subjective health complaints in pseudoneurology; thus, the prevalence of anxiety and depression should be used cautiously. Second, this study was cross-sectional, which would not enable us to understand causal relations between dependent and independent variables. Finally, it is not impossible that the control data included some asymptomatic COVID-19 patients, which may have influenced the study results. Despite these limitations, this study found some pieces of evidence for future studies on the association between COVID-19 and neuropsychological disorders.
Conclusion
This study demonstrated a remarkably high prevalence of PNCs, thus predicting upcoming neuropsychological health concerns among the community during and after the COVID-19 pandemic. This study also indicated that individuals with lower household income were significant victims of COVID-19. Healthcare amenities must be prepared to address these issues and mitigate the current and upcoming health burden of PNCs. The underprivileged population should be prioritized when discussing health issues among patients with post-acute COVID-19. Additional surveys are required to monitor the health of patients with post-acute COVID-19.
Abbreviations
| aORs: |
adjusted odds ratios |
| BDT: |
Bangladeshi taka |
| CI: |
confidence interval |
| COVID-19: |
coronavirus disease 2019 |
| PNCs: |
pseudoneurological complaints |
| SD: |
standard deviation |
Declarations
Acknowledgement
The authors are thankful to the participants for providing the information used to conduct the study.
Author contributions
MA: Conceptualization, Writing—original draft, Project administration, Methodology, Investigation, Data curation, Formal analysis, Validation, Supervision, Writing—review & editing. ASB and TM: Validation, Supervision, Project administration, Writing—review & editing. All authors contributed to manuscript revision, read and approved the submitted version.
Conflicts of interest
Not applicable.
Ethical approval
The ‘Ethical Review Committee (ERC) of Uttara Adhunik Medical College’ cleared the ethical issue for this study (Approval number: UAMC/ERC/Recommend- 11/2021).
Consent to participate
Formal written informed consent was obtained from all the participants before data collection to collect, analyze, and publish their data.
Consent to publication
Not applicable
Availability of data and materials
Data will be made available on request.
Funding
Not applicable.
Copyright
© The Author(s) 2023.