Affiliation:
1Department of Cardiovascular Surgery, Xijing Hospital, Air Force Medical University, Xi’an 710032, Shaanxi, China
Affiliation:
2Department of Anesthesiology, Xi’an Children Hospital, Xi’an 710032, Shaanxi, China
Email: 27891533@qq.com
ORCID: https://orcid.org/0000-0002-7562-7620
Explor Med. 2023;4:380–392 DOI: https://doi.org/10.37349/emed.2023.00149
Received: January 28, 2023 Accepted: March 07, 2023 Published: June 30, 2023
Academic Editor: Hua Su, University of California, USA
Enhanced recovery after surgery (ERAS) is a recommended surgical strategy at present, the core content is to reduce perioperative stress response and postoperative complications through perioperative multi-mode analgesia and intensive surgery. Electroacupuncture (EA) has been widely used in various clinical applications, and its efficacy and safety have been fully proven. The application of acupuncture in ERAS will have an important impact on rehabilitation research and development. In this review, the molecular mechanism of EA in ERAS are summed up from promoting perioperative efficacy to improving postoperative immune status. The combination of EA and ERAS may better promote the recovery of patients and the development of rehabilitation.
Enhanced recovery after surgery (ERAS), also called fast-track surgery, was first proposed by Kehlet and Wilmore [1] in 2002. A number of multi-center studies have demonstrated that ERAS may significantly shorten the length of hospital stay, reduce the incidence of postoperative complications, and have significant advantages over traditional methods in terms of costs [2, 3]. The advantages of ERAS are primarily in optimizing perioperative treatments and updating surgical procedures. For example, the update of preoperative indications, the optimized selection of intraoperative anesthesia, the rational use of the nasogastric tube and drainage tube, and the popularized application of minimally invasive surgical techniques. Compared with traditional perioperative management, ERAS has shown great advantages in reducing hospital stays, costs, stress response, postoperative pain, and the period of recovering intestinal function. Therefore, ERAS is essentially an innovation of treatments in perioperative periods based on evidence-based medicine, and the traditional surgical viewpoints are corrected [4, 5]. However, ERAS lacks a proactive technology to address several perioperative problems. While ERAS emphasizes a multi-model and multi-disciplinary approach to solving the relevant problems, the use of drugs may lead to adverse reactions that interfere with patients’ recovery. To solve the problems above, scholars have focused on the important role of perioperative acupuncture medicine [6–8].
Acupuncture is often regarded as a hallmark of traditional Chinese medicine (TCM), which may achieve analgesic effects by regulating the nervous system and the release of peptido-based neurotransmitters [6]. Electroacupuncture (EA) is performed on the basis of traditional acupuncture, which is combined with acupuncture and electric stimulation technology, and the therapeutic effect has been confirmed by various clinical and experimental studies [7]. EA is performed on the basis of traditional acupuncture, and is mainly to stimulate acupoints through the needle tail electrify, so as to achieve the purpose of treatment. Studies have demonstrated that EA has unique advantages for patients, which are beyond the scope of traditional anesthesia [8]. Researchers showed that preoperative acupuncture can relieve anxiety, optimize preoperative status, and reduce the use of anesthetics, while postoperative acupuncture may help intestinal function recovery, pain management, reduce postoperative nausea and vomiting (PONV), and shorten hospital stay [8]. This review aims to claim the molecular mechanisms of EA in the following aspects of ERAS (analgesia, reduction of PONV, postoperative anti-inflammatory, and maintenance of immune function, etc., Figure 1).
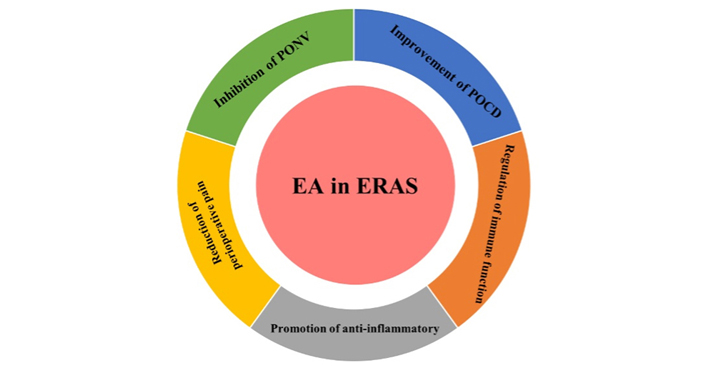
EA, to improve perioperative complications, mainly acted on five aspects: reduction of perioperative pain, inhibition of PONV, improvement of postoperative cognitive dysfunction (POCD), regulation of immune function, and promotion of anti-inflammatory
Pain is one of the major postoperative stress factors for patients, which may lead to early postoperative ambulation, delay of discharge, and it can postoperative rehabilitation, and affect the postoperative quality of life (QoL) of patients. Therefore, multi-mode analgesia is a very important part of ERAS. Multi-mode analgesia is the combined application of various methods to reduce the dose of opioids and their adverse reactions. As the main drug for postoperative analgesia, opioids are prone to cause intestinal paralysis, PONV, and other complications, and reducing their dose is conducive to the early recovery of patients [9–12]. Burnstock [13, 14] proposed the original hypothesis that acupuncture increases endogenous opioid analgesia and provided evidence for the involvement of purine receptors in acupuncture analgesia. Recently, studies have shown that EA treatment for promoting functional recovery after spinal cord injury and electric stimulation enhances the proliferation and migration of fibroblast cells [15, 16]. In this part, the mechanism of reducing perioperative pain are discussed from five aspects (Figure 2).
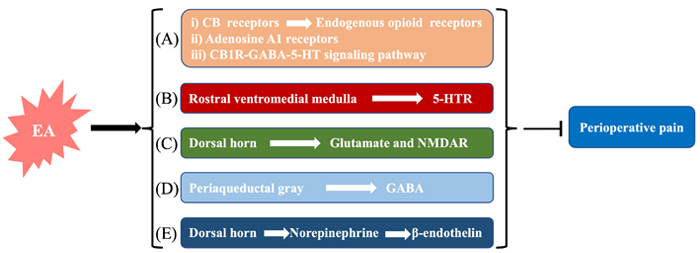
The molecular mechanisms of perioperative pain reduction. (A) i) EA activates cannabinoid receptors (CBRs) to upregulate endogenous opioid receptors to relieve pain; ii) EA activates adenosine A1 receptor to relieve pain; iii) after EA stimulation, the CB1R-GABA-5-HT signaling pathway is activated to upregulate the expression of endogenous opioid receptors; (B) EA may upregulate the expression of 5-HT1R, 5-HT2R, 5-HT3R, 5-HT4R, and 5-HT7R; (C) the activation of glutamate (Glu) and N-methyl-d-aspartate receptor (NMDAR) promotes patients’ recovery; (D) GABA is closely associated with EA stimulation in reducing perioperative pain; (E) EA can downregulate pain inhibition pathways by enhancing the release of norepinephrine to achieve the analgesic effect. GABA: γ-aminobutyric acid; 5-HT: 5-hydroxytryptamine; 5-HT1R: 5-HT receptor 1
EA stimulation releases endogenous opioids for active pain management [17, 18]. The receptors are also involved in pain regulation [19]. The endogenous opioid mechanism is the most well-known neuronal mechanism of analgesia. There are mainly four opioids: enkephalin, endorphin, strong enkephalin, nociceptive receptors, nocicin peptide receptors, δ-, μ-, and κ-opioid receptors [20]. Elucidation of endogenous responses to pain is essential to promote therapeutic effects and reduce side effects [21]. Different frequencies of EA may trigger different endogenous opioid mechanisms, and the recovery of low-frequency EA is better than that of high-frequency EA [22]. A large number of studies have shown that endogenous opioids and neuropeptides play an important role in the release of the central nervous system (CNS), and their release is due to the electrical stimulation of a certain part at a specific frequency, which then causes a series of physiological effects [22, 23]. Han [24] believes that the analgesic mechanism is related to the frequency of EA. In his study, 2 Hz EA stimulation in Zusanli (ST36) activated endorphins and bound to μ- and δ-opioid receptors, while 100 Hz EA activated endorphins and bound to κ-opioid receptors, and 15 Hz EA triggered endorphins and strong endorphins to relieve pain, so low-frequency EA is more effective in relieving pain [24]. In the treatment of inflammatory pain, the activation of peripheral CB2R may increase the level of β-endorphin in inflammatory tissues and combine with the activation of µ-opioid receptors to achieve analgesia [25]. Activation of the µ-opioid receptor may reduce the expression level of netrin-1 to alleviate neuropathic pain [26]. Yuan et al. [27] suggested that EA could relieve pain associated with cannabinoids (CBs). The endocannabinoid system is mainly composed of G protein-coupled receptors (CB1R and CB2R), endocannabinoid ligands, endocannabinoid synthesis enzymes, and degradation enzymes, which are widely distributed in CNS. Zhang et al. [28] showed that EA may relieve acute inflammatory pain by activating CB2R. Yuan et al. [27] found that EA could increase the expression of CB1R levels in the midbrain. Therefore, they suggested that EA can improve diffuse noxious inhibitory control (DNIC) function and control chronic pain through a novel CB1R-GABA-5-HT signaling pathway [29]. In addition, EA plays an analgesic effect by activating the adenosine A1 receptor located on ascending nerve and releasing adenosine [30]. Mast cells convert mechanical stimulation into EA signals by activating histamine H1 or adenosine A1 receptors, thus mediating EA analgesia [31, 32]. Therefore, based on the clinical approaches of ERAS, EA analgesia may be used to better control wound pain and reduce the dose of analgesics including opioids, thus speeding up the recovery of patients.
5-HT is often called serotonin, it is a neurotransmitter involved in the descending inhibitory system of the brainstem for analgesia. 5-HT is a peripheral pain mediator that may reduce the secretion of pain-related factors after EA [33]. It is mainly produced in the rostral ventromedial medulla (RVM) of the spinal cord and plays a bidirectional regulatory role in the down-promoting and inhibitory pathways [34]. The secretion of 5-HT in the brain increases during EA stimulation [35]. Studies have shown that 5-HT1R and 5-HT2R can be up-regulated by EA to relieve surgical pain [36]. It also alleviates surgical pain by downregulating immune activity [substance P (SP), natural killer 1 (NK-1), and cyclooxygenase-1 (COX-1)] and endocannabinoid expression levels. Related experiments showed that 5-HT1R and 5-HT3R may mediate EA analgesia, and the 5-HT2R is conversely involved in the nociceptive response [37]. Liu et al. [38] found that levels of 5-HT in RVM and trigeminal caudate nucleus were higher in the EA treatment groups than in other groups in the rat model of recurrent migraine. After EA treatment, the number of neurons and the relative protein expression of 5-HT7R were significantly reduced in migraine rats [39]. Ketotryptamine pretreatment blocked the analgesic effect of EA but did not affect the weight-bearing of rats in the sham EA control group. Electrical stimulation also activates serotonergic nucheus raphe magnus (NRM) neurons that project to the spinal cord. These results suggest that EA inhibits osteoarthritis-induced pain by enhancing spinal 5-HT2AR/5-HT2CR activity [40]. In addition, Liu et al. [41] measured the levels of 5-HT and 5-HT4R in chronic visceral hypersensitivity rats by enzyme-linked immunosorbent assay (ELISA), and the results suggested that EA could increase the pain threshold, decrease the level of 5-HT and increase the level of 5-HT4R. The study above clearly showed that 5-HT is involved in EA stimulation [41].
Glu is the most widely transmitted neurotransmitter in CNS. It is released from the spinal dorsal horn and plays a crucial role in the excitatory pathway [42]. Glu and its receptor, NMDAR, are involved in the transmission and integration of pain information at the spinal level [20]. Glu may induce CNS sensitization by activating its receptors as analgesics. EA can achieve an analgesic effect by down-regulating Glu in ascending excitation pathway [34]. Centrally sensitized NMDAR may be involved in the pain of the spinal cord [43]. Zhang et al. [44] evaluated the potential additive and/or synergistic effects of EA and sub-effective doses of diazoxepine maleate (MK-801), a noncompetitive NMDAR antagonist, on hyperalgesia in the same rat model of inflammatory pain. The results showed that antagonists of EA combined with NMDAR produced stronger anti-hyperalgesia effects [44]. The effect of wrist-ankle acupuncture (WA) stimulation at “R4”-“R5”-“R6” on the expression of Glu and phosphorylated protein NMDAR1 (p-NMDAR1) of the spinal dorsal horn in spared nerve injury (SNI) rats were observed. As a result, pain sensitivity may be reduced by inhibiting the expression of Glu and p-NMDAR1 in the spinal dorsal horn [45]. The above studies indicated that the regulation of Glu and its receptors by EA is conducive to the development of analgesic effects [34, 43–45].
GABA mainly acts as an inhibitor in the CNS. The analgesic mechanism of EA is closely related to GABA expression. It is well known that GABAA and GABAB are involved in pain regulation [46]. GABA-mediated signaling is involved in EA improvement of pain in mice experiment with knee arthritis [27]. The periaqueductal gray (PAG) is one of the main centers of the downward pain inhibition system [47]. GABA released in PAG may be involved in pain management [48]. GABA may be involved in compensatory enhanced acupuncture analgesia. After EA treatment, increased GABA has an analgesic effect on patients, which indicated the somatosensory component of acupuncture modulated primary somatosensory functional connectivity associated with insular neurochemistry to reduce pain severity in fibromyalgia (FM) [49]. In addition, EA seems to up-regulate the expression of GABA and its receptors in the spinal cord, thus providing a good analgesic effect for rats with neck pain after incision [50]. Potassium chloride cotransporter 2 (KCC2) and GABAA receptor γ2 subunit contribute to natriuretic peptide (NP) following peripheral nerve injury, C2 subunit of the GABAA receptor is involved in EA to relieve neuropathic pain [51]. Other types of studies have demonstrated that EA may induce endogenous endorphin release, and inhibit GABA release by activating μ-opioid receptors in GABAergic neurons [52]. Therefore, GABA is involved in the pain management of EA, and the frequency and area of EA may also affect GABA production.
Studies have shown that norepinephrine combined with the α2-adrenergic receptor may achieve an analgesic effect [53]. The mechanisms through which EA and tricyclic antidepressants produce analgesia seem to be complementary: EA inhibits the transmission of noxious messages by activating supraspinal serotonergic and noradrenergic neurons that project to the spinal cord, whereas tricyclic antidepressants affect pain transmission by inhibiting the reuptake of norepinephrine and serotonin at the spinal level. Zhang et al. [54] demonstrated that EA may down-regulate pain inhibition pathways by enhancing the release of norepinephrine. EA controls the transmission of pain information by activating the projection of norepinephrine neurons in the spinal cord [55]. The analgesic effect of norepinephrine on the spinal dorsal horn may be through the activation of the inhibitory factor α2-adrenergic receptor [56]. Norepinephrine involvement in pain relief is mediated by stimulating adrenergic receptors on inflammatory cells that release β-endothelin for analgesic effects [57]. In addition, there may be cross-tolerance between norepinephrine and EA, which may be due to the massive secretion of norepinephrine in the brain, acting through α receptors to counter EA analgesia [58].
In recent years, EA has been shown to not only plays an important role in relieving perioperative pain but also has an impact on relieving PONV, improving POCD, regulating immune function, and exerting an anti-inflammatory effect. The molecular mechanisms of the above aspects are described in detail.
PONV refers to nausea and vomiting occurring within 24 h after surgery, and some studies have pointed out that the incidence of PONV is 20–80% [59]. PONV not only reduces perioperative QoL, but also makes it difficult for patients to recover, and prolongs hospital stay and increases costs. The molecular mechanism of PONV is mainly related to two aspects, one is the nerve conduction pathway of PONV, and the other is the various receptors and neurotransmitters related to PONV. The emetic center is located in the medulla oblongata. Meanwhile, the chemoreceptor emetic area in the brain stem and the higher central margin participate in the process of vomiting. After all sorts of stimulation pass into the vomiting center, the vomiting center sends out impulses, producing effect effector to vomiting through the sympathetic nerve, thus, vomiting can produce. Through these related neurotransmitters or receptors (such as 5-HT, histamine, choline), the signal of PONV can be transmitted, and then treatment of PONV requires blocking neurotransmitters or receptors to prevent them from taking effect. In recent years, EA has become one of the most popular methods for remission of PONV. Studies have shown that EA can prevent PONV mainly by affecting the release of opioids, the transport of serotonin, and the regulation of the autonomic nervous function of the digestive system [23, 60]. EA for the treatment of PONV may be due to the stimulation of different acupoints [such as Hegu (LI4), Neiguan (PC6), and ST36] to stimulate the immune function, which may capture the changes of the nervous system in certain areas of the brain (Figure 3). EA stimulation may promote the release of endorphins, then endorphins dissolve δ-opioid receptors which regulate emetic action, so as to achieve the effect of antiemetic (Figure 4) [23, 60]. EA can obviously prevent the occurrence of PONV, but the mechanism needs to be further explored.
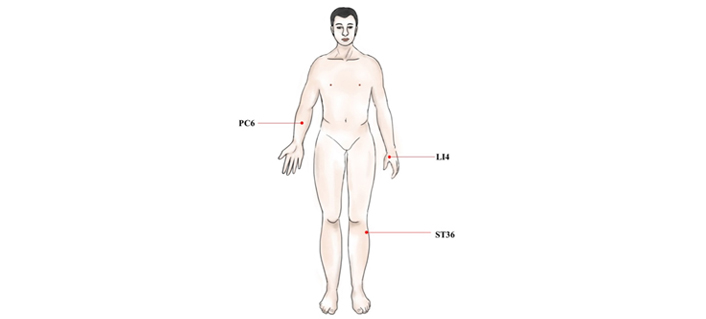
The main acupoints act in the PONV. PC6: this point is at 2.0 cun above the wrist stripes, between the palmaris longus tendon and flexor carpi radialis tendon; LI4: this point lies between the 1st and the 2nd metacarpal bones. The point is depressed like a valley; ST36: the point is on the legs, 3.0 cun below the knee
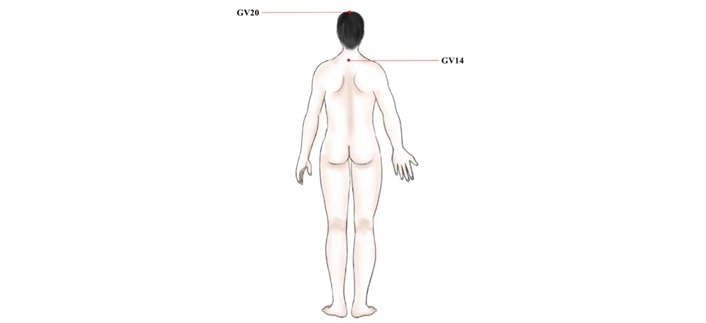
The main acupoints to improve POCD. GV20: this point is located at the vertex where three Foot-yang meridians, liver meridian, and Governor Vessel meet; GV14: this point is in the spinous process of the 7th cervical spine. GV20: Baihui; GV14: Dazhui
The delayed recovery of POCD is inconsistent with ERAS [61, 62]. In recent years, studies have shown that EA can significantly improve POCD, inhibit neuronal apoptosis, and reduce oxidative stress and inflammatory response caused by myocardial ischemia-reperfusion [63]. Some studies also found that stimulation of GV20 improved POCD caused by propofol [64]. EA ameliorates cognitive impairment induced by prolonged propofol anesthesia, which may be related to phosphorylated glycogen synthase kinase-3β (pGSK-3β) levels in CA1 independent of opioid receptors. In addition, EA stimulation of GV20 and GV14 showed improvements in cognitive function after left lobectomy (Figure 4). The results of behavioral tests showed that EA significantly improved POCD, possibly down-regulating the level of proinflammatory cytokines [tumor necrosis factor-α (TNF-α), interleukin 1β (IL-1β), and IL-6)] in the hippocampus through Toll-like receptor 2 (TLR2) and TLR4 signaling pathway in microglia (Figure 5) [65]. To further investigate the effects of GV20 and GV14 on the expression of angiotensin II (Ang II) and Ang II-type I receptor (ATIR) in the hippocampus of D-galactose-induced aging rats after POCD. The results showed that EA could improve POCD after partial hepatectomy in aging rats induced by D-galactose. The underlying mechanism may be related to inhibiting the positive expression of Ang II, ATIR, and ATIR messenger ribonucleic acid (mRNA) in the hippocampus [66]. EA may obviously achieve the improvement of POCD, but the specific mechanism needs to be further studied.
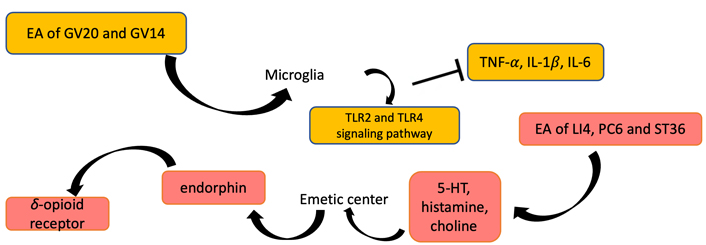
EA stimulation can be functioned in inhibiting PONV and improving cognitive function. EA of LI4, PC6, and ST36 regulates the levels of 5-HT, histamine, and choline, so as to stimulate the emetic center. Then, endorphins and δ-opioid receptors can be promoted to release. EA of GV20 and GV14 lead to the levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α) and TLR2 and TLR4 protein expression decreased
Perioperative stress response refers to the complex interaction between the nervous, endocrine, immune, and coagulation systems when patients are strongly stimulated during perioperative anesthesia. It is important to use anesthetic and non-anesthetic measures in the perioperative period to reduce harmful stress responses. Anesthetic and surgical stimulation may lead to decreased immune function and adversely affect the long-term prognosis of postoperative patients. Therefore, how to reduce and control perioperative stress and trauma and improve prognosis is the core content. EA may relieve postoperative pruritus, promote postoperative immune function, and improve the long-term prognosis of patients. A study found that stimulation of bilateral LI4, PC6, and ST36 in the patients who had breast surgery reduced the incidence of postoperative itching (2.61%) compared with the control group (8.25%), and the levels of TNF-α and IL-8 were down-regulated (Figure 6) [67]. Further, the incidence of postoperative side effects was all lower in the EA stimulation group [67]. Moreover, many immune cells, such as macrophages, NK cells, and lymphocytes, participate in the regulation of the immune system (Figure 7A) [67, 68]. In addition, Liang et al. [69] divided 29 craniotomy patients into three groups and detected TNF-α, IL-8, and IL-10, respectively. They found that EA may improve the immune function suppressed by the surgery and regulate the immune balance of patients who underwent supratentorial craniotomy [69].
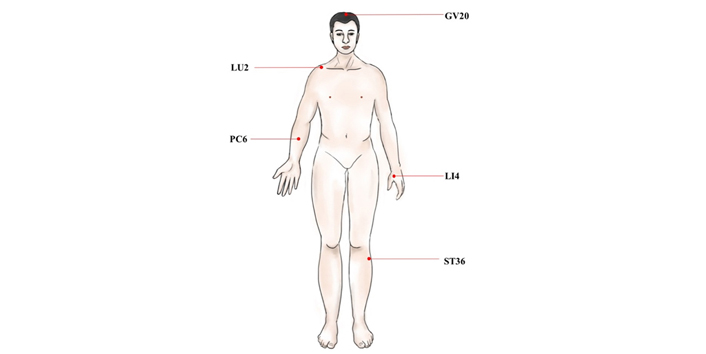
The main acupoints to regulate immune function and promote anti-inflammatory. LU2: this point is located in the subclavian fossa, in the inner margin of the coracoid process of the scapula, 6.0 cun apart from the anterior midline. LU2: Yunmen
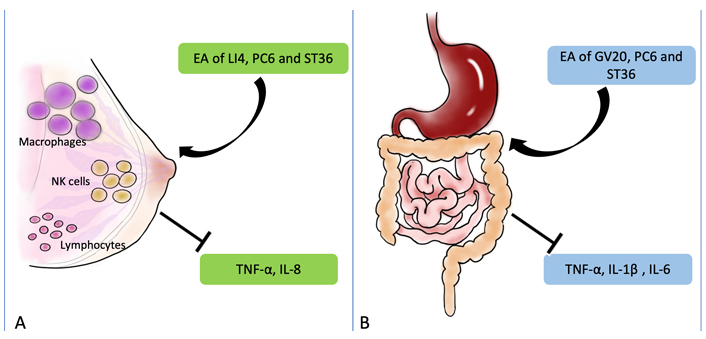
The molecular mechanisms of EA to treat other perioperative complications. (A) After EA treatment, the immune function may be regulated in patients underwent supratentorial craniotomy, which resulted from the regulation of macrophages, NK cells, and lymphocytes; (B) EA may improve the function of anti-inflammatory. EA of GV20, PC6, and ST36 can down-regulated the levels of TNF-α, IL-1β, and IL-6. The black arrow and vertical symbol represent promotion and prevention, respectively
Anesthesia and surgical stimulation may reduce the immune function, which is not conducive to the long-term prognosis of patients after surgery. Studies have shown that the levels of TNF-α, IL-2, and IL-10 changed after EA stimulation in PC6 and LU2 [70]. The results showed that EA down-regulated the levels of TNF-α and up-regulated the levels of IL-2 and IL-10 [70]. Under the same level of anesthesia, surgery can partially improve perioperative immunosuppression [70]. Studies have confirmed that 30 min after general anesthesia, EA stimulation in GV20, PC6, and ST36 after colorectal cancer surgery significantly reduced the incidence of POCD and inhibited the release of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 (Figure 7B) [71]. A recent study found that dopamine inhibits cytokine production and controls inflammation [72]. They hypothesized that EA may be an alternative strategy to vagal nerve stimulation, which can control systemic inflammation by inducing the vagal nerve to activate the aromatic carbamate decarboxylase, leading to dopamine production in the adrenal medulla [72]. Therefore, the anti-inflammatory effect of EA may be induced by the vagal nerve to activate inflammatory L-amino acid decarboxylase, thereby producing dopamine in the adrenal medulla [72].
In this review, EA is introduced and have proved to be an excellent treatment for ERAS. It focuses not only on the surgeries but also on the prognosis as well as perioperative diagnosis and treatment. However, EA still has the following disadvantages: (1) the selection of different points will lead to different effects, and the molecular mechanisms between points remain to be clarified; (2) the mechanisms of different neurotransmitters should be demonstrated further; (3) the disease spectrum which may be treated by EA is not clear, and the molecular mechanisms need to be further studied; (4) the design of experiment should be improved to avoid bias possibly.
With the development of ERAS, the application of EA in the perioperative period will have more benefits in patients’ postoperative recovery. However, whether perioperative EA can affect the long-term postoperative health status of patients still needs further research, so as to enhance the theoretical significance of perioperative EA and expand its clinical application. In the next decade, we believe EA will become an integral part of ERAS through the continuous improvement of EA in clinical applications.
5-HT: 5-hydroxytryptamine
5-HT1R: 5-hydroxytryptamine receptor 1
Ang II: angiotensin II
ATIR: angiotensin II-type I receptor
CBRs: cannabinoid receptors
CNS: central nervous system
EA: electroacupuncture
ERAS: enhanced recovery after surgery
GABA: γ-aminobutyric acid
Glu: glutamate
GV14: Dazhui
GV20: Baihui
IL-1β: interleukin 1β
LI4: Hegu
NK-1: natural killer 1
NMDAR: N-methyl-d-aspartate receptor
PC6: Neiguan
POCD: postoperative cognitive dysfunction
PONV: postoperative nausea and vomiting
ST36: Zusanli
TLR2: Toll-like receptor 2
TNF-α: tumor necrosis factor-α
YM: Writing—original draft. LY: Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The original contributions presented in the study are included in the article material, further inquiries can be directed to the corresponding author (27891533@qq.com).
This research was supported by
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2421
Download: 33
Times Cited: 0
