Affiliation:
1Department of Radiobiology and Molecular Genetics, VINČA Institute of Nuclear Sciences-National Institute of the Republic of Serbia, University of Belgrade, 11000 Belgrade, Serbia
ORCID: https://orcid.org/0000-0002-4769-2652
Affiliation:
1Department of Radiobiology and Molecular Genetics, VINČA Institute of Nuclear Sciences-National Institute of the Republic of Serbia, University of Belgrade, 11000 Belgrade, Serbia
ORCID: https://orcid.org/0000-0002-5486-0079
Affiliation:
2Department of Endocrinology and Diabetes, Zemun Clinical Hospital, Faculty of Medicine, University of Belgrade, 11000 Belgrade, Serbia
ORCID: https://orcid.org/0000-0001-6371-6610
Affiliation:
1Department of Radiobiology and Molecular Genetics, VINČA Institute of Nuclear Sciences-National Institute of the Republic of Serbia, University of Belgrade, 11000 Belgrade, Serbia
ORCID: https://orcid.org/0000-0002-3005-7943
Affiliation:
1Department of Radiobiology and Molecular Genetics, VINČA Institute of Nuclear Sciences-National Institute of the Republic of Serbia, University of Belgrade, 11000 Belgrade, Serbia
Email: isenovic@yahoo.com
ORCID: https://orcid.org/0000-0002-0012-2636
Explor Med. 2023;4:576–588 DOI: https://doi.org/10.37349/emed.2023.00162
Received: May 04, 2023 Accepted: May 29, 2023 Published: August 31, 2023
Academic Editor: Jun Ren, Fudan University Zhongshan Hospital, China
The current literature findings on autophagy’s beneficial and detrimental roles in diabetes mellitus (DM) and diabetes-related comorbidities were reviewed. The effects of oral hypoglycaemic medicines and autophagy in DM. Autophagy plays an important function in cellular homeostasis by promoting cell survival or initiating cell death in physiological settings was also assessed. Although autophagy protects insulin-target tissues, organelle failure caused by autophagy malfunction influences DM and other metabolic diseases. Endoplasmic reticulum and oxidative stress enhance autophagy levels, making it easier to regulate stress-induced intracellular changes. Evidence suggests that autophagy-caused cell death can occur when autophagy is overstimulated and constitutively activated, which might prevent or develop DM. Even though the precise role of autophagy in DM complications is uncertain, deregulation of the autophagic machinery is strongly linked to beta cell destruction and the aetiology of DM. Thus, improving autophagy dysfunction is a possible therapeutic objective in treating DM and other metabolic disorders.
Diabetes mellitus (DM) is an endocrine disease caused by defects in insulin secretion or actions and characterized by hyperglycaemia [1, 2]. The pandemic proportions of DM, with over 425 million people estimated to have DM worldwide, threaten human health [3, 4]. Furthermore, according to the World Health Organization, the prevalence of DM is in a constant upward trend, especially worrying that DM is in the range of the most common causes of death [5]. Apart from genetic predisposition, the high-risk factors for DM development are an unhealthy diet, a sedentary lifestyle, obesity, and other bad habits [6, 7]. Cardiovascular complications arose due to DM-caused cell and tissue damage and dysfunction of various organs [8–10]. Although all types of DM are treatable and DM pathology research has come a long way, the exact mechanism of pathological processes that trigger the onset of DM and further development of DM-related complications still needs to be clarified. Evidence suggests that autophagy may be a critical regulatory signaling pathway in DM development and prevention [11, 12].
Autophagy plays a crucial role in cellular homeostasis in physiological conditions by promoting cell survival or initiating cell death [13]. Autophagy is a housekeeping catabolic process that enables the removal of excess or damaged cellular components and organelles and regulates normal cell function, including pancreatic beta cells [14, 15]. In addition, autophagy protects cells in different stress conditions, including ischemia and hypoxia, oxidative stress (OxS), hyperglycemia, hyperlipidemia, and other harmful factors [16, 17]. However, dysregulation of autophagic machinery is tightly linked with beta cell injury and the pathophysiology of DM [12]. In addition, evidence shows that autophagy restoration allows a protective effect, but over-activation of basal autophagy may cause cell death [18]. Thus, the particular role of autophagy in diabetic complications still needs to be elucidated.
This review discusses recent literature on the role of both the protective and detrimental effects of autophagy in DM and DM-related complications.
From 1978 to April 2023, the MEDLINE and PubMed databases for all English and non-English articles containing an English abstract were searched. All of the publications found, and all of the abstracts from national and international cardiovascular symposia into one thorough evaluation were combined. Search terms were DM, autophagy, autophagy and endoplasmic reticulum stress (ERS), autophagy and OxS, DM and autophagy, autophagy and anti-hyperglycemic.
DM is one of the most common endocrinological diseases, with a constantly rising prevalence. Besides, chronic high blood glucose affects fat, protein, and carbohydrate metabolism due to inadequate insulin secretion, insulin effects, or both [19]. DM can have long-term consequences on a person’s health, leading to damage to numerous organs, among which the eyes, kidneys, heart, and blood vessels are most often affected [9, 20]. DM is a consequence of the modern lifestyle and the increase in other factors, among which obesity stands out [21, 22].
There are several main types of DM: type 1 DM (T1DM), type 2 DM (T2DM), gestational DM (GDM), and specific types of DM caused by different factors [19]. Several pathological processes have been shown to influence the development of DM thus far [23].
T1DM occurs in fewer people diagnosed with DM, usually 5–10% of all DM cases. T1DM is also known as insulin-dependent DM or juvenile DM. This ailment is caused by T cell-mediated autoimmune disease, which is defined by the destruction of pancreatic cells, the absence of insulin, and hyperglycemia [20, 24]. The destruction of beta cells of the pancreas in the younger population is usually fast and can lead to ketoacidosis, sometimes the initial presentation of the disease. In others, the function of the beta cells of the pancreas can be preserved enough to prevent the occurrence of ketoacidosis [23, 25].
T2DM is prevalent in most people diagnosed with DM, accounting for 90–95% of all DM cases. T2DM is the most common metabolic disorder. This type of DM is often called insulin-independent DM and affects people who have insulin resistance (IR) or insulin deficiency [19]. With this type of DM, where the peripheral cells resist endogenous insulin, insulin administration is unnecessary to survive [24]. Most patients with T2DM are obese, which affects the development of IR, or have a distinct abdominal type of obesity. Hyperglycemia can develop for years, and the disease remains unnoticed until severe symptoms that indicate the presence of DM develop [26, 27]. Both macro and microvascular complications often occur in patients with T2DM [28]. The risk of T2DM increases with age, obesity, and reduced physical activity [27].
In addition to these two primary types of DM, it is also important to mention GDM, which occurs in women during pregnancy and is characterized by hyperglycemia. GDM occurs due to insufficient secretion of insulin to compensate for metabolic stress caused by IR. Risk factors for developing GDM are obesity, history of previous GDM, older women, polycystic ovary syndrome, and others. Hyperglycemia often continues even after childbirth, indicating the presence of T2DM [29].
Specific types of DM include genetic disorders of pancreatic beta cells, genetic disorders of insulin action, diseases of the exocrine pancreas, neonatal DM, and DM caused by various drugs and chemicals, viruses, and others [23].
It is also worth noting the possible link between Alzheimer’s disease (AD) and DM since AD has been dubbed “type 3 DM” [30]. The exact form of the link is quite complex and needs to be fully known; however, certain essential aspects connect AD with DM, particularly T2DM. DM is linked to an increased incidence of dementia and cognitive decline, both of which are symptoms of AD [31, 32]. DM management and a healthy lifestyle may have potential benefits in lowering the risk of cognitive decline and dementia [33]. Age, obesity, high blood pressure, high cholesterol levels, and a sedentary lifestyle are all risk factors for these diseases. Insulin plays an important role in the brain, and changes in insulin signaling or decreased insulin sensitivity may impact brain function and contribute to the pathology of AD [34]. DM is well-known for its adverse effects on blood vessels, resulting in vascular damage and decreased blood flow to the brain [32, 35]. Vascular issues are also linked to an increased risk of dementia and the development of AD [36, 37].
Autophagy represents a mechanism that is involved in cellular homeostasis and defence. It is a catabolic process that degrades and recycles intracellular elements. Hence, autophagy is responsible for pathogen clearance, cellular recycling, and preventing cell toxicity due to the accumulation of damaged organelles and proteins [38–40]. Besides, autophagy enables energy production since it disassembles lipids into free fatty acids that could be oxidized via mitochondria [41]. The autophagy process is linked with apoptosis and, therefore, participates in the cell cycle regulation and maintains genomic stability [42, 43]. Autophagy is classified into three categories based on how substances are transported to the lysosome: macroautophagy, microautophagy, and chaperon-mediated autophagy (Figure 1). Microautophagy and macroautophagy can be selective or non-selective (as shown in yeast and advanced organisms), although chaperon-mediated autophagy is exclusively found in mammalian systems [44]. The macroautophagy process implies a double-membrane autophagosome that surrounds the components from the cytosol, which further fuses with the lysosome. All the contents from the autophagosome are revealed to the lysosome (autolysosome) for recycling and deterioration. Microautophagy has different molecular mechanisms, and it is categorized into three forms: type 1 includes lysosomal (vacuolar) membrane protrusion, type 2 involves lysosomal (vacuolar) invagination, as well as type 3 represents the invagination of the endosomal membrane [38, 44, 45]. During chaperon-mediated autophagy, targeted proteins from the cytosol are degraded, not organelles, and there is no configuration of autophagosome and autolysosome. The protein complex is transported into the lysosome via heat shock cognate 70 kDa protein (HSC70) and lysosome-associated membrane protein type 2A (LAMP-2A). The proteins for degradation have pentapeptide KFERQ-like motif, which HSC70 recognizes, and together, they form a compound. Finally, the present complex cooperates with the cytoplasmatic tail of LAMP-2A, and the proteins for degradation are transported into the lysosome [38]. Of these three types of autophagy, macroautophagy is the most examined.
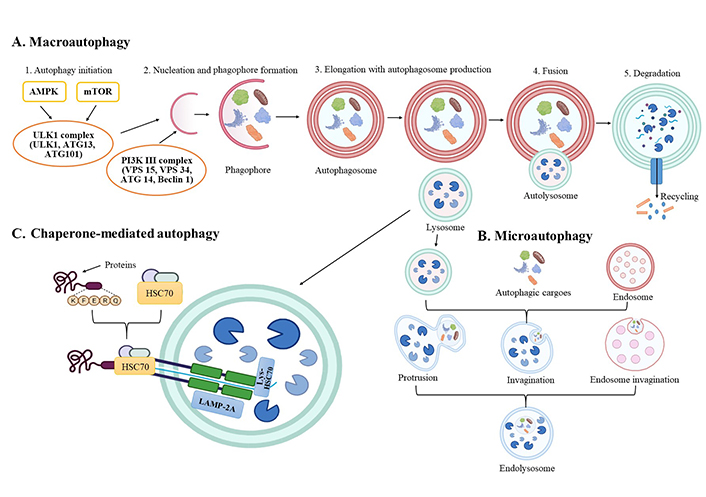
Presentation of autophagy general molecular mechanisms with three main forms. (A) Macroautophagy; (B) microautophagy; (C) chaperon-mediated autophagy. AMPK: adenosine monophosphate-activated kinase; ATG: autophagy-related genes; Lys: lysosome; mTOR: mammalian target of rapamycin; PI3K: phosphatidylinositol 3-kinase; ULK1: unc-51-like autophagy activating kinase 1. Created via BioRender.com
According to nutritional status, there is non-selective and selective autophagy. Non-selective autophagy implies stress and nutrient malnourishment, while selective autophagy of organelles, proteins, or pathogens occurs in nutrient-rich circumstances [38, 46, 47]. Selective autophagy preserves cell homeostasis and prevents the incidence of some diseases by preserving the number of organelles—mitochondria (mitophagy), peroxisomes (pexophagy), proteins (aggrephagy), lysosomes (lysophagy), and ribosomes (ribophagy) [46, 48, 49].
The main cellular signaling pathway responsible for physiological autophagy is PI3K/protein kinase B (AKT)/mTOR [50]. ATG are the most important regulators of autophagy molecular processes and signaling pathways [49, 51]. The macroautophagy process has five stages: initiation, nucleation, elongation with autophagosome production, fusion, and destruction [52, 53]. In the initiation stage, the ULK1 complex and the class III PI3K complex 1 are included. ULK1 is regulated by the mTOR complex 1 (mTORC1) and AMPK, and under starvation circumstances, in the initiation phase, mTORC1 is deactivated, resulting in the repression of ULK1 complex [53, 54]. After the initiation phase, the membrane expands, named a phagophore [55, 56]. Nucleation of the phagophore, i.e. nucleation phase, is followed by ULK1 complex phosphorylation of the PI3K complex, which stimulates phagophore formation [56]. The PI3K complex is regulated by proteins that interrelate Beclin 1 [38]. Elongation of the phagophore is supported by two ubiquitin-like protein complexes-ATG12-ATG5-ATG16L1 and ATG4B-ATG7-ATG3, and they mediate the activation of microtubule-associated protein 1A/1B-light chain 3 (LC3) into LC3I, lipidation with phosphatidylethanolamine (PE) to form LC3-PE conjugate (LC3II) [53, 57, 58]. Phagophore enlarges and eventually creates autophagosome whose dimension differs among organism types [59, 60]. The cytoplasmatic microtubular network is important in autophagosome fusion with the lysosome/vacuole, especially syntaxin 17 (STX17), vacuolar morphogenesis protein 7 and 9, and vesicle-associated membrane protein 8 [53, 61]. In the degradation phase, autolysosome contents are exposed to the acidic lumen, degraded by lysosomal hydrolases, and released back into the cytoplasm through lysosomal permeases for cell reuse [56, 62].
Autophagy has an important function in cellular physiological and pathological regulation and viability. Therefore, autophagy is associated with cell/tissue injuries due to systematic disorders, hypoxia, starvation, infection, etc. It is speculated that correct autophagy control could improve DM-related systemic problems [63]. DM is disposed to cellular alterations accelerated by autophagy [64]. According to the literature, autophagy has double-edged sword functions in DM pathology since, in the beginning, it reduces ERS and consequently protects the cells [65].
Nonetheless, systemic activation of autophagy might not always stimulate beneficial properties for non-targeted healthy cells. With the DM pathology progress, impaired autophagy misplaces its protective role and participates in the development of several DM chronic complications (Figure 2), including diabetic retinopathy, cardiomyopathy, vasculopathy, nephropathy, neuropathy, and skeletal alterations [46, 66–68]. Besides, the dysregulation of autophagy could be used as a possible therapeutic target in DM [69]. Therefore, autophagy should be monitored at every disease stage of all target organs [70]. Understanding the role of autophagy in cellular maintenance is currently the focus of interest, with the help of various molecular laboratory techniques [71]. Moreover, clarifying the autophagy specifics generated by different disorders in all cells is essential.
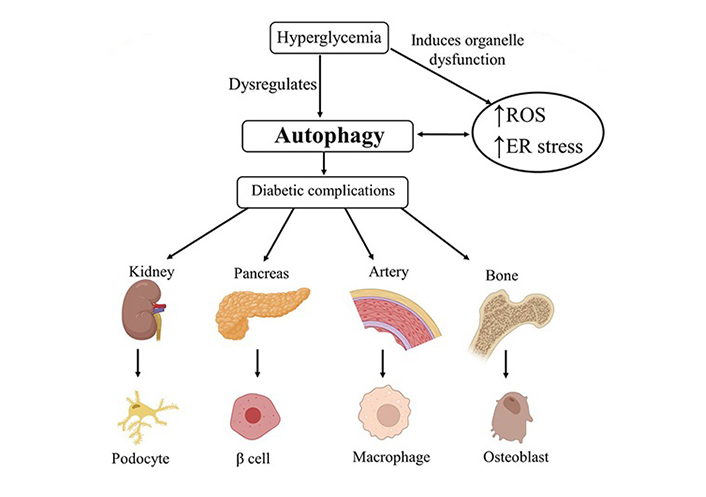
Impact of imbalanced autophagy in the development of diabetic complications in different tissues. ROS: reactive oxygen species; ER: endoplasmic reticulum. Created via BioRender.com
For cells to survive and function properly, continual intracellular synthesis must occur, and degradation is necessary [72]. DM and other metabolic diseases are influenced by various organelle dysfunctions caused by deranged autophagy [43, 73]. Low autophagy levels regulate survival and function, protecting insulin-targeting tissues [11, 74]. The ERS and OxS increase autophagy levels, controlling stress-induced intracellular changes [14, 43, 75–77]. Autophagy-caused cell death can, however, result from excessively stimulated and constitutively activated autophagy [78–81].
In diabetic animal models, genetic or chemical inhibition of autophagy may cause acceleration of beta cell mass and function loss [11]. Both sexes’ T1DM and T2DM animal models exhibit decreased autophagy [77, 82–89]. Defective lysosomes and reduced insulin secretion are associated with abnormal autophagy in T1DM female and male mice [82, 85]. Compared to pancreatic cells from people without DM, those with T2DM show more autophagic vacuoles and autophagosomes and express lower lysosomal genes [90, 91]. Lipotoxicity, OxS, ERS, and inflammation induce autophagy impairment, organelle damage, and faulty lipid and protein accumulation in β cells and insulin-target tissues, promoting IR and T2DM [77, 92]. The resulting glucotoxicity impacts the continued escalation of OxS and ERS and enhances inflammation [11, 77, 93, 94]. Additionally, due to an improper response to the ERS caused by obesity, autophagy deficiency in β cells may trigger the transition from obesity to DM [95]. Although mechanisms are not entirely understood, autophagy may impact the chronic vascular complications of DM [43]. Autophagy is mediated by a variety of mechanisms in the chronic complications of DM, including mTORC1 (an autophagy inhibitor), AMPK (an autophagy activator), OxS, and ERS [96–98]. In Figure 3, the three major autophagy signaling pathways—mTORC1, AMPK, and silent information regulator T1 (SIRT1) inhibition for negative autophagy regulation. AMPK activates the ULK1-ATG13-focal adhesion kinase family interacting with protein 200 complex, while mTORC1 inactivates. SIRT1 activates the ATG5-ATG7-LC3 complex and forkhead box O3 (FoxO3) [96–98].
One possible preventive and therapeutic target for DM is the derangement of autophagy [69, 82]. Therefore, tapering to sufficient autophagy is a viable therapeutic goal in treating many metabolic abnormalities [77]. Resveratrol and rapamycin are two autophagy activators shown to improve beta cell activity in diabetic animal models [11]. Two current techniques for altering autophagy in DM animal models include using autophagy inhibitors or deleting ATG [43].
Activating AMPK and SIRT1 signaling by metformin and sodium-glucose cotransporter-2 (SGLT-2) inhibitors may promote autophagy [90, 99, 100]. Metformin’s therapeutic effect on DM-related autophagy regulation is linked to the transcription factor EB (TFEB) [101]. In vitro lipotoxicity results in metformin promoting autophagy in beta cells and inhibiting their apoptosis [98]. It has been observed that the SGLT-2 inhibitor empagliflozin and the dipeptidyl peptidase 4 (DPP-4) inhibitor linagliptin restore glomerular and cardiomyocyte autophagy in db/db male mice and Zucker Diabetic Fatty male rats [102, 103]. Thiazolidinedione member, pioglitazone, promotes autophagy via the AMPK pathway [104]. Alogliptin (DPP-4 inhibitor) induces autophagy through a mechanism that depends on the SIRT1/AMPK/mTORC1 cascade [105]. Liraglutide, a member of glucagon-like peptide 1 receptor agonists, may increase autophagy in male Sprague Dawley rats, a model of chronic renal failure, by modulating the AMPK and mTORC1 pathways [106, 107]. Additionally, glucagon-like peptide 1 receptor agonists promote autophagy by reducing T2DM impairment of autophagosome-lysosome fusion [77].
There is now strong evidence that autophagy plays a role in the etiology of DM. The development of drugs that take advantage of autophagy’s putative cytoprotective effect in DM is a potentially intriguing field of research. Future research should clarify contradictory findings, and important questions about the role of autophagy dysfunction in developing IR and T2DM remain unanswered. If the pathogenic role of dysregulated autophagy in the development of DM is proven, a new class of drugs based on a novel concept will be conceivable.
Because there are multiple perspectives on the relationship between autophagy and DM, it is critical to identify specific molecular targets within the autophagy pathway that could lead to the development of novel therapeutic interventions for DM, such as strategies to enhance autophagy in specific cell types, like as pancreatic beta cells, to improve their function and survival. Furthermore, several dietary interventions activate autophagy and enhance insulin sensitivity; therefore, understanding the appropriate dietary strategies that can modify autophagy to prevent or manage DM is critical.
Understanding the relationships between autophagy regulation and other therapeutic methods and combining autophagy-targeting interventions with existing DM treatments such as insulin therapy or anti-hyperglycemia medicines, will be critical in designing effective combination therapies.
AD: Alzheimer’s disease
AMPK: adenosine monophosphate-activated kinase
ATG: autophagy-related genes
DM: diabetes mellitus
ERS: endoplasmic reticulum stress
GDM: gestational diabetes mellitus
IR: insulin resistance
LC3: microtubule-associated protein 1A/1B-light chain 3
mTOR: mammalian target of rapamycin
mTORC1: mammalian target of rapamycin complex 1
OxS: oxidative stress
PI3K: phosphatidylinositol 3-kinase
SIRT1: silent information regulator T1
T1DM: type 1 diabetes mellitus
T2DM: type 2 diabetes mellitus
ULK1: unc-51-like autophagy activating kinase 1
SZ, ZG, and JR: Writing—original draft, Conceptualization. ERI and MO: Investigation, Writing—original draft, Supervision. All authors contributed to manuscript’s revision, and read, and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was funded by the
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
