Abstract
Type 1 diabetes is a chronic condition that results from the destruction of insulin-producing β-cells in the pancreas. Current treatments for type 1 diabetes, such as insulin therapy and pancreatic islet transplantation, have several limitations and, hence not quite effective in the long run. As current therapy methods fail to slow disease development, novel strategies such as the development of a bioartificial pancreas are being seriously considered. Over the last decade, research has focused on tissue engineering, which aids in the design of biological alternatives for the repair and replacement of non-functional or damaged organs. Three dimensional (3D) bioprinting technology which employs 3D printing technology to generate 3D tissue-like structures from biomaterials and cells, offers a promising solution for the treatment of type 1 diabetes by providing the ability to generate functional endocrine pancreatic tissue. Bioprinted structures are therefore an important aspect of tissue engineering because they have been found to replicate the native extracellular matrix, promoting cell survival and proliferation. In this review, recent developments in 3D bioprinting of endocrine pancreas for the treatment of type 1 diabetes particularly focussing on the choice of cells, biomaterials, growth factors, and essential considerations have been discussed in detail. Additionally, the key challenges and perspectives towards recapitulation of the pancreatic function of the pancreatic organ engineering technologies have also been discussed.
Keywords
Biomaterials, 3D bioprinting, pancreas, islets, type 1 diabetes, biofabricationIntroduction
Anatomy of pancreas and the islets of Langerhans
The pancreas is a glandular organ situated in the abdominal region, extending behind the stomach towards the left upper abdomen near the spleen [1]. The pancreas is anatomically divided into four parts: the head (extends from the duodenum), the body (stretches behind the stomach), the neck, and the tail (extends near the spleen) (Figure 1) [1]. This organ has an extensive arterial blood supply, with the celiac artery and superior mesenteric arteries being the major blood vessels that enable so [2]. Ischemia to the pancreas arising from vascular obstruction is uncommon due to this dual blood supply. The pancreas is a heterocrine gland, i.e., it performs both endocrine and exocrine functions [3]. Its primary function as an exocrine gland is to secrete pancreatic juice into the duodenum via the pancreatic duct. This juice contains bicarbonate, which neutralizes stomach acid that enters the duodenum, as well as digestive enzymes, which help in the digestion of the food that enters the duodenum from the stomach. Most of the pancreatic tissue is associated with exocrine functions and comprises two types of cells, namely: 1) the pancreatic acini, comprising of multi-lobed pyramidal-shaped cells known as acinar cells, and 2) the ductal cells, which are cuboidal cells lining and branching from the acini outwards feeding into the common bile duct. The acinar cells synthesize and secrete the exocrine (digestive) enzymes into the pancreatic duct, whereas the ductal cells release the pancreatic enzymes to the digestive system [4]. The rest of the pancreas functions as an endocrine gland by regulating the blood sugar levels and energy metabolism by secretion of specific hormones. The pancreas consists of specialized cells known as the pancreatic islets or ‘islets of Langerhans’ which are actively involved in the production of hormones such as insulin, glucagon, somatostatin, and pancreatic polypeptide [5].
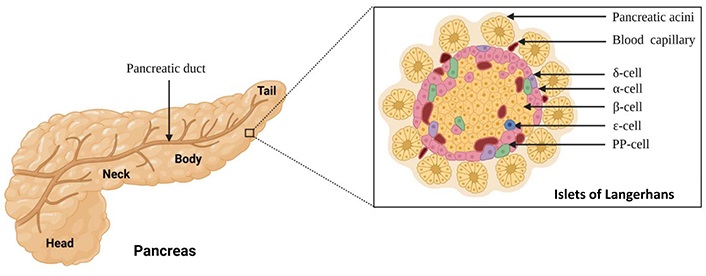
Schematic of anatomical regions of the pancreas along with the structure of a single pancreatic islet with the different cell types present within. PP-cell: pancreatic protein cell
Note. Adapted from “Pancreatic islet of Langerhans”, by BioRender.com (2023). Retrieved from: https://app.biorender.com/biorender-templates. Used under BioRender’s Academic License Terms
The “islets of Langerhans” are named after German pathologist Paul Langerhans, who discovered them in 1869. They are structured aggregates of endocrine cells evenly distributed throughout the pancreas and roughly constitute 1% of the total weight of the pancreas. The islets exist as complex micro-organisms made up of various cell types that contribute to the release of endocrine hormones and peptides that are associated with blood glucose homeostasis [6]. Morphologically, human islets are spherical or oval three dimensional (3D) clusters whose size usually varies from 50 µm to 500 µm in diameter [7]. Human islets are composed of five different cell types: α-cells, β-cells, δ-cells, ε-cells, and PP-cells [8, 9]. Each islet gets nourished by an extensive microvascular network to ensure the cells receive adequate nutrients and oxygen. The α-cells make up approximately 15–20% of the pancreatic islets and are responsible for the synthesis and secretion of the peptide hormone glucagon. Glucagon helps in mobilizing stored glycogen into glucose to prevent hypoglycaemia in healthy individuals [10]. The β-cells form the main part of the pancreatic islets and account for 60–70% of the mass of the pancreatic islets. These cells are involved in the production and release of another peptide hormone, insulin, into the blood. Insulin regulates blood glucose levels and is responsible for the storage of glucose in the liver, muscles, and adipose tissue [11]. Insulin and glucagon work together to achieve the common goal of regulating blood glucose levels where insulin acts through an anabolic pathway and glucagon through a catabolic pathway. The δ-cells, which account for 5–10% of the islet mass, release the peptide hormone somatostatin, which plays a regulatory role in inhibiting the release of glucagon and insulin from the α-cells and β-cells, respectively [12]. The ε-cells constitute less than 1% of the pancreatic islets and are involved in the production of ghrelin, the hunger-inducing hormone [13]. The PP-cells are the least well-studied islet cells and are responsible for the production of pancreatic polypeptides. They account for less than 5% of pancreatic islet cells and have been shown to have effects on gastrointestinal motility, act as a satiety factor, and have some metabolic effects, including suppression of insulin and somatostatin secretion [14]. The cytoarchitecture of pancreatic islets has been reported to vary between species [15]. While rodent islets are characterized by a preponderance of insulin-producing β-cells in the nucleus and a lack of α-cells, δ-cells, and PP-cells in the periphery, human islets have α-cells and β-cells in close proximity throughout the cluster [8, 9]. There are few studies on the structure of human islet cells, and a clear description of their cellular organization is lacking. There is agreement on the different endocrine cell types, which do not differ significantly between rodent and human islets, and on the proportion of islet cells, which is lower in humans than in rodents [8, 16]. However, the cellular arrangement of endocrine cells in human islets is still a matter of debate. Although human islets have often been depicted with a simple nucleus-mantle architecture like rodent islets, there are reports that have described human islets with a different cellular organization [17–19]. The most common description of islet cell composition and arrangement comes from research in rats and mice. It is generally believed that the endocrine cells are not randomly distributed in the islets. In most rodents, β-cells form the core of islets, whereas non-β-cells such as α-cells, δ-cells, and PP-cells form the mantle. This unique architecture appears to have some functional implications. All islets have a dense capillary network that is five times denser than its exocrine counterpart. This ensures that the highly metabolically active cells receive an adequate supply of oxygen and nutrients, while allowing the cells to respond rapidly to changes in blood glucose levels [20]. Sympathetic fibers are also located within the islets, allowing autonomic innervation of the islets [21].
A brief overview of type 1 diabetes
Diabetes mellitus (DM) is a chronic metabolic condition characterized by abnormally high blood glucose levels (hyperglycemia), which leads to long-term problems in a variety of organs, including nephropathy, neuropathy, retinopathy, and vasculopathy. It’s a major public health concern worldwide that costs both the healthcare system and the world economy a lot of money. By accounting for approximately 1.5 million deaths directly and even more indirectly due to hyperglycemia-related complications per year, it is the 8th leading cause of death worldwide [22]. DM can be classified into numerous types, including type 1 DM (T1DM), type 2 DM (T2DM), maturity-onset diabetes of the young (MODY), gestational diabetes, and neonatal diabetes [23]. T1DM is also known as insulin-dependent diabetes or juvenile diabetes. A recent meta-analysis revealed that the global prevalence of T1DM is approximately 9.5% with 15 out of 100,000 individuals reportedly suffering from this condition [24]. It also constitutes about 5–10% of the diabetic patients making it one of the most common subtypes of DM [25].
T1DM is a chronic autoimmune disorder characterized by hyperglycemia caused due to insulin deficiency following the destruction of β-cells of the pancreatic islets [26]. It’s one of the most prominent endocrine and metabolic disorders in children. T1DM-related autoimmunity which is concurrent with the development of T1DM-associated auto-antibodies causes the loss of β-cells in the great majority of patients (70–90%). These patients have autoimmune T1DM also known as type 1a DM [26]. Alternatively, there are no immune responses or auto-antibodies discovered in a smaller group of patients, and the cause of cell damage is unknown. This form of DM has a strong hereditary component and is known as idiopathic T1DM or type 1b DM [27]. Although symptoms normally appear during childhood or early adolescence, they can sometimes appear much later. Although the aetiology of T1DM is unknown, the pathophysiology of the illness is considered to entail T cell-mediated destruction of β-cells. Islet-targeting auto-antibodies that target proteins associated with secretory granules in β-cells such as insulin, 65 kDa glutamic acid decarboxylase (GAD65), insulinoma-associated protein 2 (IA2) or zinc transporter 8 (ZNT8) are biomarkers of T1DM-associated autoimmunity that can be used to identify and investigate individuals at risk of developing T1DM [28, 29]. The type of auto-antibody that develops initially is determined by both environmental and hereditary variables. The pathophysiology of T1DM has been proposed to be a continuum that commences with the detection of auto-antibodies and progresses to β-cell death, dysglycemia, and, eventual emergence of symptoms associated with hyperglycemia [30]. Polyuria, polydipsia, polyphagia, weight loss, hazy eyesight, and excessive weariness are all symptoms of T1DM [26]. T1DM can strike at any age, however, it is more frequent in children and young people.
Current treatment options towards management of T1DM
The basic idea behind the management of this condition is to keep the blood sugar level as close to normal as possible to delay or prevent complications. The current status of T1DM treatment includes extensive blood glucose monitoring supplemented by exogenous administration of insulin through multiple injections daily [31]. Although these treatments can slow the advancement of diabetic problems such as nephropathy and retinopathy, they are insufficient to prevent them. Despite medical advances, there has yet to be developed an insulin treatment that can imitate physiological rhythms or a mechanical substitute for the β-cells of the pancreas [22]. An alternative option to insulin administration is replacing the endocrine mass by transplanting allogeneic pancreas or pancreatic islets. It is worth mentioning that despite having higher costs involved due to multiple surgical procedures, pancreas transplantation is currently more commonly employed in clinical practise as compared to islet transplantation. However, pancreas transplantation is currently restricted for individuals with advanced stages of T1DM and severe or end-stage kidney failure. Furthermore, the surgical process is linked with considerable mortality risk, as well as clinically relevant consequences such as pancreatitis, hemorrhage, re-occurrence of autoimmunity, and post-transplantation rejection, necessitating the urgent quest for an alternative therapeutic strategy [32]. Transplanting the damaged cells is a practical alternative to complete pancreas transplantation. Pancreatic islet transplantation is a simple, minimally invasive procedure in which purified allogenic donor islets are recovered from a deceased organ donor and percutaneously injected into the recipient’s liver via the portal vein. In the recipient’s body, these islets begin to produce and release insulin [33–35]. More than one injection of these transplanted islet cells is required to avoid dependency on externally administered insulin. This treatment offers lesser risk to the patient compared to pancreatic transplantation since no major surgery is necessary. For many decades, the effective use and the potential of islet transplantation as a therapy for T1DM was not acknowledged until diabetes reversal was first reported in rats and in a patient suffering from chronic pancreatitis who underwent pancreatectomy followed by islet transplantation [36, 37]. Following these results, much study in the field of islet transplantation has been done. Despite tremendous advancements in islet transplantation techniques, major barriers to practical application still remain. The current clinical paradigm of treatment is infusing islets into the patient’s liver via the portal vein, where they confront a suboptimal non-pancreatic environment characterized by high glucose concentrations, low oxygen tension, and a greater level of toxins [38]. Furthermore, islet infusion through the hepatic portal vein causes an immediate blood-mediated inflammatory reaction [39]. Additionally, hypoxic islets produce chemokines and express tissue factors, triggering a thrombotic response, following which platelets that are drawn to the islet surface, attract leukocytes and macrophages, which infiltrate and kill the islet cells [40]. Cumulatively these factors destroy up to 70% of the transplanted islets within 48 h, thereby limiting islet engraftment, and hampering the success rate of the procedure [41]. As a result, islets from up to three donors are required for clinically desirable outcomes, thereby drastically limiting the availability of the transplant due to a shortage of donors. A possible alternative xenotransplantation of pancreatic islets has been suggested as a solution to the donor scarcity. Nevertheless, due to xenogenic immunological rejection, the xenotransplanted islets are severely rejected [24]. Another significant obstacle to islet transplantation is the requirement to take an immunosuppressive medication. Immunosuppressive medications have several adverse effects, including an increased risk of infection, a greater rate of malignancy, hepatotoxicity, and nephrotoxicity all of which greatly reduce the patient’s quality of life [42]. As a result, pancreatic islet encapsulation is being actively researched to minimize the dose of immunosuppressive drugs. Islet encapsulation involves utilizing biomaterials that serve as an immunoprotective barrier to avoid the need for immunosuppressive medications in safeguarding the transplanted islets [24].
Islet encapsulation and the need for 3D bioprinting technology
The pancreas is known to be the islets’ natural habitat and therefore has extensively been investigated as a potential location for islet transplantation. Unfortunately, due to metabolic problems such as pancreatitis and restricted vascular supply, it is not regarded as a transplantation site. Since no viable alternative transplantation site in the human body has been reported as yet, one possibility to consider is the fabrication of an artificial transplantation site [24]. Recent breakthroughs in bioengineering technology now permit the fabrication of such sites. To this end, hydrogels have been used as biomaterial scaffolds that may be introduced to imitate tissue-like qualities. The main approach to incorporating such technology in islet transplantation is via encapsulation of the pancreatic islets. Pancreatic islets are embedded within a hydrogel network, which functions as a “bioartificial pancreas” allowing bidirectional diffusion of small molecules approximately 6 kDa in size, such as insulin, oxygen, nutrients, and glucose while also safeguarding islets from immune attack by restricting access to immune cells or antibodies which are larger in size (approximately 150–900 kDa) (Figure 2) [43]. Numerous small animal models have shown that islet encapsulation can potentially improve glucose homeostasis from a short-term perspective, however, no lasting restoration of euglycemia has been found [44–46]. Several obstacles reportedly prevent this biological approach from progressing into clinical settings. Conventional encapsulation techniques have significant drawbacks, including hypoxia, degradability, reproducibility, scalability, and retrievability. A major issue emerges as a result of physical defects in the fabrication of the hydrogel, resulting in an insufficient encapsulation of the islets. This may culminate into pericapsular fibrotic overgrowth (PFO), which limits nutrition and oxygen passage, causing islet necrosis [47, 48]. Even effectively encapsulated islets suffer from hypoxia because of the restricted hydrogel permeability and greater distance from neighboring blood arteries, which lowers oxygen availability through diffusion. This complicates scaling up to a big animal model or therapeutically meaningful dosage of islets. Furthermore, encapsulation hinders fast re-vascularization after transplantation, exposing the islets to further hypoxic stress [24].
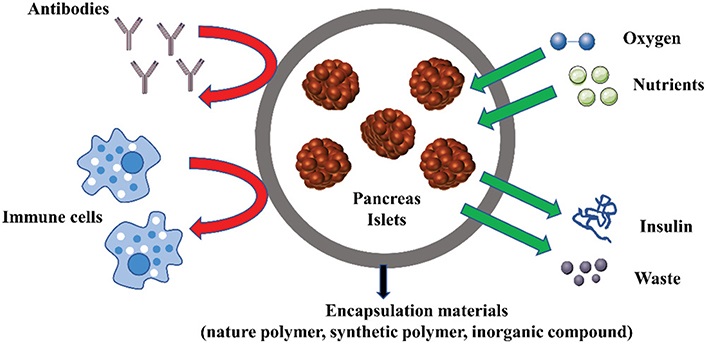
Schematic depicting biomaterial-based strategies for islet encapsulation. Encapsulation methods can protect transplanted islets from immunological response while allowing oxygen, nutrients, insulin, and metabolic waste to diffuse
Note. Reprinted with permission from “Advances in encapsulation and delivery strategies for islet transplantation,” by Wu S, Wang L, Fang Y, Huang H, You X, Wu J. Adv Healthc Mater. 2021;10:e2100965 (https://onlinelibrary.wiley.com/doi/epdf/10.1002/adhm.202100965). © 2021 Wiley-VCH GmbH.
One strategy for addressing hypoxia is the “seeding” islets into degradable 3D scaffolds. Such scaffolds can be fabricated using biomaterials that are capable of providing a microenvironment similar to the native pancreas by employing 3D bioprinting. In essence, 3D bioprinting involves the deposition of a bioink comprising cells, biomaterials, and occasionally growth factors in layers layer-by-layer manner. Such automated printing enables the exact control of architecture, pore interconnectivity, and great repeatability required for commercial clinical use and regulation [24]. Furthermore, bioprinting enables the exact deposition of a diverse range of cell types and bioactive substances to mimic native tissue environments and encourage cell survival. When compared to hydrogels, the bioprinting scaffolds offer more precise designing, and better spatiotemporal distribution of encapsulated cells, besides providing a higher surface area to volume ratio to promote vascularization, oxygen and nutrient diffusion. The extracellular matrix (ECM) proteins are deposited by surrounding tissues and engrafted islets as the scaffold slowly degrades, gradually rebuilding the proper environment necessary for islet survival [49]. Although immunological isolation cannot be established with this strategy, the scaffolds can limit the direct interaction of implanted islets with circulating immune cells, hence reducing the inflammatory response until the scaffolds degrade [39, 50]. Furthermore, as mentioned earlier, it allows highly regulated seeding of islets, which can help reduce the development of PFO caused by conventional approaches. The aim of this review is to present the most recent advances in islet encapsulation using 3D printing technology, and the development of 3D bioprinted pancreas towards the management of T1DM. Additionally, the current challenges and future perspectives which could benefit further research in this domain are also highlighted.
3D bioprinting and types of bioprinting
Driven by tissue engineering, 3D bioprinting technology is a subset of additive manufacturing, which involves the fabrication of artificial tissues and organs for biomedical applications. Recently, 3D bioprinting technique has emerged as a novel bio-fabrication technique that may be used to create highly complex tissue models with regulated porosity geometry and excellent reproducibility [51, 52]. In essence, bioprinting involves the computer-assisted designing (CAD) and process buildup used for carrying out the spatiotemporal, layer-by-layer deposition of biological materials known as bioinks into 3D structures resembling tissues and organs. The lack of biological structures needed to regenerate damaged organs and tissues is the primary driving force behind the inception of 3D bioprinting. The ultimate goal is to offer a suitable substitute for tissue implants and animal testing practices used in illness research and the development of medicines [53]. At present, the application of 3D bioprinting is restricted to the in vitro fabrication of tissues and organs for evaluating drug efficacy, nevertheless, 3D bioprinting has enormous potential for substituting lost or failed organs in patients. 3D bioprinting is more difficult than conventional 3D printing due to the involvement of cells, which are exceedingly sensitive and require special care to allow them to grow and divide while avoiding the cytotoxic activity of the materials and conditions used during the process. The advancement of approaches that facilitate the fabrication of functional living structures in order to restore the functions of tissues and organs is the primary objective of 3D bioprinting.
Tissue engineers have come up with strategies for the fabrication of artificial tissues exhibiting structural and functional recapitulation of their native counterparts. 3D bioprinting relies on three broad approaches: biomimicry, autonomous self-assembly, and mini-tissue building blocks [54]. The most common approach to bioprinting is known as biomimicry involves drawing knowledge from nature and applying it towards the fabrication of structures that almost mimic the natural tissues and organs found in vivo in terms of structure, organization, and microenvironment. This approach necessitates precise reproducibility of specific cellular functional components of tissues. This is achieved through a thorough understanding of the microenvironment, including cell type arrangement, ECM composition, a gradient of soluble and insoluble factors, and the behaviour of biological factors. The second bioprinting approach is autonomous self-assembly, which is a method of replicating biological tissue that follows the developmental biology route i.e., embryonic tissue and organ development. The cells create their own ECM building blocks, cell signaling, and self-orchestrated patterning during their early development to provide the necessary biological functions and micro-architecture. During the process, a scaffold-free version is formed using self-assembling cellular spheroids that undergo differentiation and organization to form the desired tissue [55]. This approach considers the cell as the basic driving force of histogenesis, directing the building blocks, structural, and functional properties of the newly formed tissue. It necessitates a greater understanding of how embryonic tissue mechanisms develop, as well as the microenvironment in which the bioprinted tissues are created. The mini-tissue building blocks approach combines both of the aforementioned strategies. Mini-tissues, which are small functional units of tissues and organs, are formed using this bioprinting method. The bioprinting process starts with the assembling of mini-tissues into macro-tissues guided by a bioinspired organization, followed by the replication of tissue units that are able to self-assemble to form functional structures [56].
Stages of bioprinting process
Three distinct steps can be taken to complete the 3D bioprinting process overall, which are pre-bioprinting, bioprinting, and post-bioprinting.
Pre-bioprinting
Pre-bioprinting involves the development of a printer-useable CAD model and the development of a bioink to be subsequently used for printing. This step conventionally involves technologies like magnetic resonance imaging (MRI) scans and computed tomography (CT) scans which provide images that are then tomographically reconstructed to produce two dimensional (2D) images. Once the design is completed in CAD, it will proceed to the slicing process, which will generate G-code for the transition to the standard tessellation language (STL) file, which can communicate with a printer [57]. This stage also encompasses the selection of biomaterial(s), and the obtaining of cells at desired densities through direct isolation from tissue biopsy, or conventional sub-culturing methods, for bioink development. To keep the cells viable, oxygen and other nutrients are combined with it. The pre-bioprinting phase is crucial in influencing the characteristics of the constructs produced during bioprinting. It is essential to follow the proper methods during the pre-bioprinting stage to guarantee that the required cell quality is obtained which can then promote tissue formation after bioprinting [58].
Bioprinting
This is the stage where the actual printing process occurs. The bioink is then loaded into the printer cartridge, which deposits the substance in accordance with the predetermined digital model developed during the pre-bioprinting stage. To create 3D tissue structures that resemble biological tissues, cell-laden bioinks are deposited in a layer-by-layer manner onto the substrate. Crosslinking is an essential event following successful bioprinting that has a substantial impact on the biomechanical stability and physicochemical properties of bioprinted constructs as well as the cellular behavior of loaded living cells [59]. This part of the bioprinting procedure is challenging since it sometimes necessitates printing using several cell types depending on the tissues and organs being created [58].
Post-bioprinting
Post-bioprinting is the final step in the bioprinting process that is necessary to give the printed structure functionality. In this phase, the tissue structure is developed in an incubator or more preferably in a bioreactor. Physical and chemical stimulations are required to maintain the structure and function. These simulations send signals to cells, triggering them to reconfigure themselves while sustaining tissue growth. Because post-processing conditions have a strong influence on cell-cell interactions, a wide range of stimuli can be used to modulate the tissue maturation process during post-bioprinting processes. The printed construct is subsequently evaluated and biologically compared to native tissue before being used for its intended purpose [58].
Types of bioprinting
Extrusion-based bioprinting
The most popular technique for printing 3D biological structures is extrusion-based bioprinting (EBB), also known as micro-extrusion. Several research groups have employed this bioprinting technology for research involving tissue engineering. EBB is considered the most commonly used bioprinting modality because of its versatility and the wide availability of biopolymers that can be extruded. In essence, EBB is a nozzle-based printing technique in which the bioinks are continuously and precisely extruded through a syringe nozzle during the printing process. The variety of material selection across a wide range of bioink and the high accuracy of production of chemically appropriate tissues or organs are the most promising aspects of EBB. The shear-thinning biopolymers, which aid in extruding the bioink, are most frequently used to create extrudable bioinks. The hydrogels, also known as printable biopolymers, are non-Newtonian fluids in which viscosity decreases under shear stress. The mechanical pressure used in extrusion straightens the entangled polymer chains, lowers the viscosity of the bioink inside the nozzle, and promotes the survival of living cells. Due to negligible shear during printing, the hydrogel regains its viscosity, providing printing fidelity to the finished construct. An extrusion bioprinter may use pneumatic, piston, or screw-based mechanical pressure, depending on the parameters of the hydrogel and the cells. The resolution, precision, and overall structural fidelity of the printing construct are significantly impacted by the extrusion pressure, nozzle diameter, and printing speed of the extrusion bioprinter. The primary process parameter of the printer for a particular hydrogel is nozzle diameter optimization. High extrusion pressure is necessary for a nozzle with a small diameter for a viscous hydrogel, and this pressure also increases the shear stress on the cells. Higher shear stresses can cause damage to the cells, which lowers the vitality of the cells after printing [60, 61]. The ability to print very viscous hydrogels with high cell concentration at a moderate speed is one of the main advantages of this technique. Additionally, a complex functioning organ can be printed utilizing a multi-head or co-axial head extrusion bioprinter, which allows the printing of numerous cell types using the same or distinct hydrogels within the same construct [62]. Without doubt, EBB is the most preferred technique when bioprinting bioartificial pancreas. Likely so majority of the studies reviewed in this article have been carried out using EBB and its variations to achieve precise bioprinted structures with high shape fidelity and complexity quite close to their native counterparts.
Inkjet-based bioprinting
Another popular technology is inkjet bioprinting, also known as drop-on-demand bioprinting, and it can be used for both biological and non-biological purposes. The ink in the cartridge was eventually changed to biological material, and the paper was replaced with an electronically controlled elevator stage to give control. Initially, this technology was exclusively utilised for 2D ink-based printing. At the moment, inkjet bioprinting may be carried out on bioprinters specially created to handle and print biological materials with great precision, speed, and resolution. This technique is based on drop-on-demand techniques. Different biosimilars are encased within the hydrogel matrix, and premixed bioink solutions are placed inside the inkjet cartridge to be depleted dropwise through the inkjet printhead in a regulated manner. The bioink chamber can produce the bioink droplets by applying either thermal energy [63, 64] or piezoelectric impulses [65, 66]. Tiny air bubbles created by thermal energy during thermal inject bioprinting create pressure pulses within the bioink solution that force out bioink droplets of various diameters. The temperature gradient, the viscosity of the bioink, and cell concentration all affect the size of the droplets. For piezoelectric inkjet bioprinting, a polycrystalline piezoelectric ceramic material transforms the applied electric current to transient mechanical pressure, causing a droplet of bioink to expel onto a construction platform. Both single and multiple piezoelectric printheads are possible. Different cell kinds can be deposited in the same printed construct at the same time using other printheads [67]. Piezoelectric-based printheads can control droplet volume more precisely than thermal printheads, making them more practical sometimes. However, because the typical operating frequency of a piezoelectric printhead is between 15 kHz and 25 kHz, which might harm the cell membrane, many researchers favour thermal inkjet bioprinting over piezoelectric technology [63]. Inkjet bioprinting has a high printing resolution of approximately 50–300 μm [68]. Nevertheless, this method takes a long time since submicrometer-sized droplet volumes are tiny. The main disadvantage of this method is that bioprinting is challenging when the viscosity of hydrogel is high (> 10 cP) and the cell concentration is greater than 5 × 106 cells/mL [69]. When the cell concentration is high, cell aggregation or sedimentation might happen in the nozzle and cause cell clogging. The hydrogels must also be sufficiently wettable and have the right amount of surface tension to pass through the cartridge and nozzle. Additionally, the printed structures obtained by this method exhibit weak mechanical integrity due to low viscosity. These factors have limited the widespread usage of this technique for the development of bioartificial pancreas.
Laser-assisted bioprinting
The process of depositing biomaterials onto a surface while employing a laser as an energy source is known as laser-assisted bioprinting (LAB). This method had previously only been used to transfer metals, but it has recently been altered to work with biological components like cells, DNA, and peptides. A layer of biological material generated in a liquid solution with a receiving substrate facing the projector, along with a ribbon with donor transport support, makes up a laser-assisted bioprinter. In LAB, biomaterials such as hydrogel, culture media, cells, proteins, and ceramic materials will be employed. LAB is a nozzle-free dispensing technique that allows for a wide range of bioink viscosity and cell concentration changes without clogging the nozzle. While the cell concentration can be increased up to 1 × 108 cells/mL without causing nozzle blockage, the bioink viscosity can be adjusted between 1 mPa·s and 300 mPa·s without impacting the printing resolution [69]. A ribbon, commonly a laser-transparent material made of either glass slide or quartz, is part of the printer arrangement. The donor side of the ribbon is coated with a laser-absorbing medium (such as Ag, Au, Ti, or TiO2) before the cell-encapsulated hydrogels are sprayed on top of the laser-absorbing coating. The absorbing media are focused by the laser impulse through the ribbon, and as they evaporate, they raise the local pressure on the bioink film. In the direction of the bioink film, cavitation-like bubbles are produced by the absorbing media’s vapour pressure. The bioink layer’s jet is formed by the bubble’s expansion and contraction, which causes the bioink droplets to be created and transmitted to the printing substrate [70, 71]. The LAB has numerous benefits, but there are also some downsides, such as the constant contamination of the printed substrate by the absorbing media. The fragile spray coating used in LAB can quickly dry on the ribbon surface before printing [72]. These bioprinters move at a medium speed, and roughly 95% of the cells are still viable after the process. However, random cell distribution results in cells spreading onto the ribbon, which results in uneven cell printing. The major limiting factors towards the widespread implementation of LAB for bioprinting include involvement of high costs and limited materials being reported which can be printed using this technique.
3D bioprinting of pancreas
3D bioprinting is a fully automated layer-by-layer additive manufacturing involving the spatiotemporal and patterned deposition of a bioink comprising cells, biomaterials, and occasionally growth factors via rapid prototyping technologies to fabricate bioartificial tissues and organs with multicellular components, hierarchical structures, and complex functions [73]. By enabling precise control over the deposition of cell-laden bioink with micrometer-level accuracy, this technique solves the issues associated with standard scaffold-based tissue engineering systems. The use of 3D bioprinting to create an artificial pancreas comprising pancreatic islets is still in its infancy. This technology typically involves dispensing bioinks encapsulating pancreatic islets within biopolymers that mimic the pancreatic microenvironment layer by layer, after which the bioprinted structures are cultured with occasional growth factor supplementation to initiate tissue functionality within bioprinted constructs (Figure 3). When compared to hydrogel capsules, the bioprinted constructs exhibit a higher surface area-to-volume ratio and even facilitate vascular ingrowth, which increases oxygen and nutrition supply [74]. The ECM proteins are deposited by surrounding tissues and engrafted islets as the scaffold slowly degrades, gradually recreating the proper environment required for islet survival and effective engraftment [75].
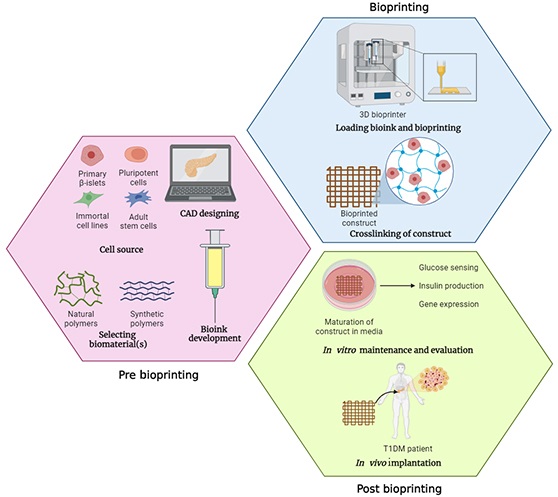
Schematic illustration of the application of 3D bioprinting technology for the development of bioartificial pancreas for patients with T1DM. Created with BioRender.com
Essential considerations in bioink development
A solution of a biopolymer or a combination of several biopolymers, generally encapsulating the desired cellular components, known as the bioink, is used to create tissue constructs during the bioprinting process. Usually, hydrogel forms are preferred for developing bioinks since they provide a highly permeable and hydrated 3D matrix conducive to cellular adherence, and subsequent migration, growth, proliferation, and differentiation [76]. Bioinks can be made entirely of natural or synthetic biomaterials, or as a fusion of the two. In some cases, cell aggregates without any additional biomaterials (scaffold-free) can be used as a bioink in bioprinting processes [77]. An ideal bioink should have the required physical, rheological, and biological properties of the target tissues, which are required to ensure the bioprinted tissues’ functionality. Bioink development and its characterization have become critical to the success of 3D bioprinting as they are used to create the final shapes of the desired tissue structures. Towards meeting this goal, the bioink needs to satisfy several parameters, including rheology, viscosity, gelation kinetics, viscoelasticity, porosity, biocompatibility, and biodegradability.
Rheological properties of the bioink play a vital role in influencing the printability, and formation of precisely controlled structural geometries having high shape fidelity, besides ensuring optimal cell viability [78]. The printability of a bioink strongly depends on multiple factors such as bioink homogeneity, crosslinking technique, viscosity, surface tension, along with the bioprinting modality used [79]. In the case of EBB, which is by far the most extensively performed approach, the extrusion pressure applied on the bioink leads to an increase in shear stress upon the bioink, possibly causing an exponential increase in cell damage thereby reducing the number of viable cells [80]. For a bioink to be printable, it needs to possess shear-thinning behaviour allowing it to be readily extruded from a nozzle without clogging and retaining its shape after deposition to create structures with great structural integrity [81]. Another important property of bioink is its viscosity, which is known to play a key role in the biofabrication of tissues. A prior study found that the viscosity of a cell-laden bioink affects the uniform cell encapsulation within the bioink and form fidelity following its deposition [82]. An optimum viscosity can assist bioinks in avoiding droplet formation due to surface tension and assist bioink towards continuous strand production, resulting in better shape fidelity and printability. Low-viscosity bioinks form strands that tend to spread throughout the substrate and collapse until crosslinking is initiated. In contrast, bioinks with high viscosity form filaments, cause nozzle clogging and are difficult to print, besides hindering oxygen, and nutrient diffusion through the polymer matrix [82]. This mandates the need to strike a fine balance in terms of material viscosity for bioprinting applications. Appropriate viscoelastic characteristics are critical in protecting the cells against structural instability and shear pressures within the bioink. The gelation kinetics and crosslinking density are interdependent parameters that govern the bioink flow ability, and shape fidelity of the construct. Additionally, beyond a certain critical value (which varies with the choice of polymer(s), and crosslinking strategy used for bioink development) it may cause localized disruption of the flow during the printing process. Crosslinking also enhances the biomechanical competency (e.g., Young’s modulus, yield stress) and reduces the degradation rate of bioprinted scaffolds regardless of chemical or microstructural properties. 3D bioprinted scaffolds are usually porous with interconnected pore networks formed due to polymer chain entanglements or crosslinking. The scaffold porosity and pore size directly affect their functioning in biological applications by modulating oxygen and nutrient diffusion to cells. Porosity is required in 3D bioprinted constructs for facilitating cell nourishment, proliferation, and migration, as well as tissue vascularization and tissue development [83]. A porous surface also helps to increase the mechanical stability of the implant by facilitating mechanical interaction between the scaffolds and surrounding tissue [84]. However, increasing porosity frequently compromises the mechanical quality that is vital in preserving the structural stability of the biomaterial. As a result, for an effective scaffold system, a balance between the mechanical and mass transport functions of the scaffolds should exist. The capability of a bioink to support normal biological processes, such as the stimulation of molecular and mechanical signaling systems, without eliciting toxic effects that can reduce cell viability or cause an immune response, is referred to as biocompatibility and forms the central basis prior to using any bioink for bioprinting applications [85]. Lastly, the polymer matrix must exhibit biodegradability, which should also need to stay tuned with the growth and matrix remodelling rate of the encapsulated cell(s) within it to mimic the tissue niche in situ successfully. All these factors taken together present researchers with a significant challenge to develop appropriate bioinks ideal for their function.
Cells: the building blocks of bioartificial pancreas
Main cells
One of the most challenging situations for researchers is the selection of source and type of cells while bioprinting them to replicate islet function and expedite neotissue formation. While there is an apprehension towards using allogenic and xenogenic sources due to their inherent immunogenic response, obtaining autologous cells may often be difficult and associated with donor-site morbidity. From a pancreas bioprinting perspective primary islets derived from allogenic or xenogenic sources have been encapsulated during bioprinting to develop pancreatic tissue. Primary islets are often recognized as the preferred cells since they are the native cells forming the pancreas, and can be obtained through a minor biopsy of the pancreas to subsequently extract islet cells. Although isolated islets are a common source for pancreas bioprinting, they have significant limitations including an additional surgical procedure to harvest them causing donor site morbidity, limited growth, and loss of insulin-producing capability during in vitro culture, are difficult to expand during culturing, and thus have low intrinsic healing capacity [86]. Basically, when islets are isolated, the ECM and islet vasculature are destroyed, which may have a deleterious impact on islet function after transplantation.
An alternative to the issues associated with primary cells is the use of immortalized cell lines closely resembling native islet function. They offer several advantages, such as they are cost-effective, robust, easy to use, providing an unlimited supply of cell sources, and bypassing ethical concerns associated with the use of animal and human primary cells. Insulinoma cell lines such as MIN6 [87], and INSE-1 [88], BRIN-BD11 [89] have been successfully used in bioprinting applications for replicating native islet function. Unlike primary cells, immortalized cell lines can replicate forever and are typically more robust and user-friendly. However, working with cell lines has a number of drawbacks, including the fact that they are genetically engineered. Moreover, variability in cultures can be brought about by genetic drift or extensive passaging of cell lines, which can lead to genotypic and phenotypic heterogeneity over time [90]. Cell lines may therefore not accurately represent primary cells and may produce inconsistent findings.
Another alternative to primary cells, induced pluripotent stem cells (iPSCs), which are obtained through cellular reprogramming of somatic cells have been investigated for their ability to form islet cells [91]. Pluripotent stem cells are the progenitors of all adult tissues throughout development. Technological improvements in differentiation protocols have facilitated the robust development of pancreatic β-cells from patient-derived iPSCs. Differentiation protocols for iPSC-derived islets primarily target the same developmental stages, beginning with the definitive endoderm and primitive gut tube, then narrowing cell fate to pancreatic and endocrine progenitors, before ultimately targeting the final differentiated β-cell. Growth factors are commonly used to modulate the pathways required for differentiation stages, with the goal of mimicking embryonic development [91]. Over the last decade, β-cell and pancreatic progenitor stem cell differentiation protocols have been meticulously developed, leading to functional iPSC-derived islets. Due to their non-invasive collection from the patient and autograft without immunomodulation, human iPSCs (hiPSCs) can be thought of as an advantageous substitute for stem cells used in regenerative medicine, without ethical considerations as in the case of applications involving embryonic stem cells (ESCs). Regardless of the fact that iPSCs have very low yield and are extremely immature, their in vitro differentiation into islets is quite challenging, in broad sense, there are technical challenges and financial concerns associated with iPSCs. Chromosome aberrations or mutations are common when reprogramming somatic cells to iPSCs. Furthermore, the formation of teratomas remains a concern when using iPSCs. Even though advances in iPSC-derived islet differentiation protocols are promising, a universal differentiation protocol that could be applied to all iPSC lines would make autologous cell therapy more feasible [91].
Adult stem cells (ASCs), on the other hand, represent a dependable alternative cell source. Compared to stem cells belonging to embryonic origin that are able to exhibit pluripotency, ASCs are multipotent and lineage-committed cells having a more limited capacity to differentiate into the various cell lineages of our body. However, there are ethical considerations regarding the use of ESCs in research due to the inevitable destruction of human embryos, thereby shifting the focus on ASCs [92]. ASCs reside in distinct niches with microenvironments that enable them to maintain an undifferentiated state and to replace specialized cells in the affected tissues that have been damaged [92]. Mesenchymal stem cells (MSCs), which are spindle-shaped cells initially misidentified as fibroblasts, are among the most widely used ASCs. The use of MSCs in tissue engineering can be supported by their active role in tissue regeneration and production of cells as a replacement for damaged and dead cells [93]. Recent studies have shown that MSCs are able to migrate to distant areas where damage has occurred and potentially offer reparative cells or produce soluble trophic factors through paracrine signalling which aids in cell survival, cell proliferation, and cell migration to augment tissue regrowth [94]. Furthermore, MSCs have anti-inflammatory and immunomodulatory properties, which enable them to reduce inflammation and restore or suppress immune cell functioning [95]. Most importantly, prior to usage in tissue engineering applications, MSCs are able to be readily cultured in vitro to optimal cell numbers over multiple passages without losing their self-renewal ability [96]. Given the functionalities listed above, the use of MSCs in pancreatic tissue engineering approaches has the potential to overcome the long-standing problem of finding a suitable cell source. Overall, MSCs are superior to other forms of stem cells in terms of ease of isolation, plasticity, and clinical translation to generate autologous cells. Although there exists no standard protocol for obtaining pancreatic β-cells from MSCs, multiple differentiation protocols reported by various research groups mention the use of a cocktail of small molecules and growth factors to guide MSC-differentiation towards pancreatic β-cells [97]. Although providing insight into these protocols is not a part of this review, the authors recommend recent reviews by Pavathuparambil Abdul Manaph et al. [97] and Wszoła et al. [98] for a comprehensive understanding of the topic.
Supporting cells
Technological advances in 3D bioprinting offer the use of multiple nozzles which enables the incorporation of different cell types at designated niches within a bioprinted scaffold to replicate the high complexity of tissues. Most often, the use of supporting cells alongside main tissue forming cells assists better neotissue development and, hence function. The most important factor influencing the effectiveness of islet transplantation is hypoxia [99]. This presents a major issue in the case of immunoprotective layer formation techniques such as encapsulation which is employed in bioprinting. Since 3D bioprinting primarily produces macroscale capsules, the issue of extravascular macroencapsulation, such as core hypoxia, happens since the cells are isolated from their surrounding blood vessels. As a result, the viability of the transplanted islets in the subcutaneous site is reduced significantly. Within the bioprinted constructs, the formation of a vascular system can be induced through the addition of supporting cells to create a relatively homogeneous blood flow. The islet of Langerhans is densely vascularized with fenestrated endothelium, which allows cells to detect blood glucose and secrete insulin into the systemic circulation [100]. Endothelial cells are also important in promoting islet function by upregulation of insulin transcription via cell-to-cell contact or humoral factor secretion [101]. Co-culture with endothelial progenitor cells, or human umbilical vein-derived endothelial cells (HUVECs), represents a promising strategy to promote vascularization within bioprinted constructs. Differentiating into endothelial cells, the building blocks of blood vessel endothelial lining these cells can undergo crosstalk with islet cells and promote insulin expression and secretion, as well as islet survival within bioprinted constructs to form fully functional biomimetic pancreatic tissue [102].
As part of the alloresponse following implantation of bioprinted tissue, recipient T-cells recognize alloantigens of the allograft, which activates recipient T-cells to evoke an inflammatory response at the implant site [103]. Such a response causes acute graft rejection in addition to chronic graft dysfunction, necessitating the administration of immunosuppressive drugs to prevent the onset of alloresponse. However, immunosuppressants are associated with negative side effects, therefore mandating the need to develop novel immunotherapies to achieve immunosuppression-free transplantation. T-cell sub-populations known as “regulatory T-cells (Tregs)” are experts in controlling and suppressing the immune system. Previously, in mouse hepatic allograft models, it has been demonstrated that Tregs play a significant role in spontaneous graft tolerance [104, 105], and there is an increase in Tregs proportion in patients who are spontaneously tolerant [106]. As a result, numerous strategies for making use of their innate abilities have been thoroughly researched. Particular benefits of adoptive transfer of ex vivo expanded Tregs include improved control over the creation and expansion of Tregs, the ability to conduct functional and phenotypic analyses prior to delivery, and more precise control over dosage and delivery timing [107]. This has shown promise in murine islet transplantation models [108, 109].
Biopolymers used for bioink development
Bioink development is a crucial element of 3D bioprinting because it creates the biomaterial scaffold to encapsulate cells and provides key signals for the synthesis of protein regulators and cytokines to create an appropriate 3D microenvironment. Due to their ability to form highly hydrated and permeable 3D polymeric structures that promote cellular anchorage and metabolism, hydrogels or their precursors are frequently favoured as bioink materials [76]. Several biomaterials capable of producing hydrogels have been investigated for their effect on the printing process and their ability to stimulate in vitro pancreatic tissue development. Recent case studies of bioink development and bioprinting pancreas using these biopolymers are discussed in detail ahead, with the benefits as well as drawbacks of each candidate summarized in Table 1.
Advantages and disadvantages of commonly used biopolymers in bioink development for bioartificial pancreas
| Biomaterial | Advantages | Limitations | References |
|---|---|---|---|
| Alginate |
|
| [110, 111] |
| Gelatin |
|
| [112, 113] |
| pdECM |
|
| [114, 115] |
| Hyaluronic acid |
|
| [116, 117] |
| PLA |
|
| [118, 119] |
| PCL |
|
| [120] |
MMP: matrix metalloproteinases; pdECM: pancreatic-derived ECM; PLA: polylactic acid; FDA: Food Drug Administration; RGD: arginine-glycine-aspartic acid; PCL: polycaprolactone
Alginate
Alginate is a naturally occurring polymeric material derived from the cell wall of brown algae. It is composed of D-mannuronic acid (M-block) and L-guluronic acid (G-block) linked through a 1,4-glycosidic bond [121]. Being an anionic polysaccharide-based biopolymer, it can be easily crosslinked using a divalent cation Ca2+, Sr2+, or Ba2+ enhancing viscoelastic and water-retention properties along with its increased cell binding affinity [122]. This makes alginate an ideal polymer for encapsulating various cell types for bioprinting applications [121, 123]. Alginate has been frequently employed for pancreatic islet encapsulation due to its ability to achieve immunological isolation. Alginate-encapsulated pancreatic islets have been successfully transplanted into people [124]. Many researchers concentrated on alginate and alginate-based hydrogel blends as coatings to produce immune-isolating encapsulation since these polymers have been proven to be non-immunogenic if suitably purified [123, 125]. Pancreatic islets have been observed to sustain their viability and functionality for a while after either macroencapsulation or microencapsulation in alginate was carried out [126]. However, capsule size and composition unquestionably affect islet function, primarily as a function of diffusion distances [127, 128]. The single islet that makes up each microcapsule is enclosed in a thin layer of polymer, reducing the distance between the islet and its surroundings while maintaining the barrier’s immunoprotective properties [129]. However, while macrocapsules may typically be recovered with ease in cases of graft failure, it is virtually difficult to recover microcapsules. Alginate has been frequently employed for pancreatic islet encapsulation due to its ability to achieve immunological isolation. However, as demonstrated in vivo, alginate is vulnerable to losing structural stability and integrity as a result of ion exchange with the environment [130]. Additionally, alginate lacks cell adhesion RGD motifs as well, which reduces cell survival due to anoikis [131].
The earliest attempt at bioprinting the complex micro-architecture of the pancreas was reported in 2010 by Xu et al. [132] using alginate/gelatin/fibrin hydrogels to encapsulate adipose-derived stem cells (ADSCs). The chemical similarity of gelatin and alginate to the ECM, while the property of fibrin regulates cell differentiation and self-organization into a functional tissue structure [133, 134]. Subsequently, when pancreatic islets were introduced at designated micro-holes within these ADSC-laden alginate/gelatin/fibrin hydrogels, the ADSCs were induced to differentiate into vascular endothelial cells and adipocytes to mimic the complex tissue structure of native pancreas. The resulting bioprinted constructs were stable for up to 8 weeks, and facilitated cell growth, organization, and differentiation. Following incubation of constructs in epidermal growth factor (EGF) induction of vasculature was confirmed by positive immunostaining for CD31 and CD34. Alongside, Oil Red O staining carried out confirmed the differentiation of ADSCs into spherical adipocytes. Assessment of whether the bioprinted constructs were capable of mimicking in vivo function was carried out by measuring dynamic insulin release patterns in response to 15 mM glucose stimulation, showing significantly higher insulin secretion in assembled bioprinted constructs compared to the control group comprising only free β-cells. These results were also supported by glucose consumption analysis, where seeding β-cells in the 3D structure significantly increased glucose consumption. This study is a significant achievement in the field of complex organ engineering, which has shown great promise in the establishment of physiological pancreatic tissue.
Alginate because of its excellent biocompatibility and lower toxicity has led to testing in a variety of 3D bioprinting applications [135]. Alginate has a favourable effect on cells, however because of its low viscosity, printing fidelity is compromised, making it difficult for bioprinted scaffolds to retain their shape after being deposited from the printer nozzle [136]. To address this issue, Duin et al. [137] encapsulated islets isolated from rat within an alginate/methylcellulose hydrogel blend to fabricate macroporous hydrogel structures via 3D bioprinting (Figure 4). The resultant bioink was printable and had a higher shape fidelity in comparison to plain alginate. Following the printing process, it was demonstrated via Live/Dead staining and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay that the islets encapsulated within the hydrogel had good viability and metabolic activity. Additionally, the ability of the islets to be able to continuously produce insulin and glucagon in response to glucose stimulation throughout the post-printing stage was reported through dithizone (DTZ) staining and immunofluorescence staining. Through this study it was demonstrated that the alginate/methylcellulose hydrogel does not interfere with the diffusion of relevant macromolecules. Additionally, neither the inclusion of methylcellulose nor the bioprinting process harms the morphology or survival of the encapsulated islets, thereby serving as proof-of-concept that it is possible to 3D plot pancreatic islets that remain functional and respond to glucose stimulation.
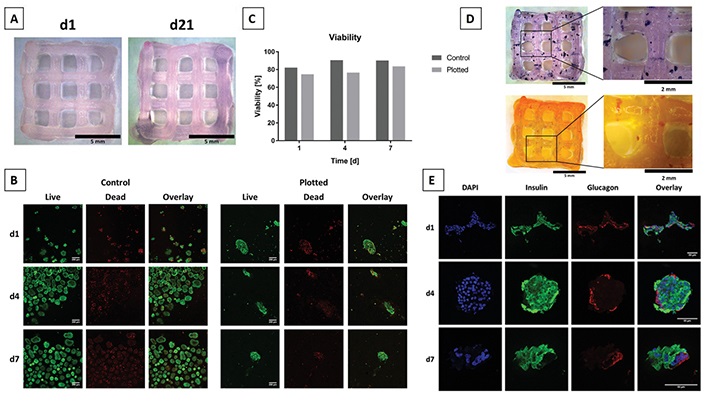
3D bioplotting islets in alginate/methylcellulose hydrogel blends. (A) Images of plotted and Sr2+ crosslinked hydrogel scaffolds demonstrating high shape fidelity and stability over 21 days in culture. Scale bars depict 5 mm; (B) live/dead staining of free islets representing the control group (left) and encapsulated islets within hydrogel scaffolds (right). Live and dead cells are shown in green and red, respectively. Scale bars depict 200 μm; (C) semi-quantitative assessment of islet viability on the basis of live/dead staining as shown in (B), n > 60 islets; (D) islets stained with MTT on day 1 after bioprinting to check for their metabolic activity (top) and islets stained for presence of insulin with DTZ on day 7 after bioprinting (bottom). Scale bars on the left depict 5 mm; scale bars on the right 2 mm; (E) immunofluorescence micrographs of encapsulated islets stained for nuclei using 4’,6-diamidino-2-phenylindole (DAPI), insulin, and glucagon incubated for 1, 4, or 7 days under cell culture conditions. Scale bars depict 50 μm
Note. Adapted with permission from “3D bioprinting of functional islets of Langerhans in an alginate/methylcellulose hydrogel blend,” by Duin S, Schütz K, Ahlfeld T, Lehmann S, Lode A, Ludwig B, et al. Adv Healthc Mater. 2019;8:1801631 (https://onlinelibrary.wiley.com/doi/10.1002/adhm.201801631). © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Recently Hu et al. [87] developed a novel bioink by adding the polymer Pluronic F127 to the alginate solution, resulting in improved the printability and flexibility of the crosslinked structure. Meanwhile, to enhance resistance against inflammatory stresses, hypomethylated pectin was added to the bioink composition without changing the viscoelastic property of the bioink. The results of the experiments demonstrated that cellular constructs printed with alginate-pluronic-pectin bioink could allow oxygen diffusion, maintain higher cell viability during exposure to the diabetogenic combination of the cytokines interleukein-1β (IL-1β), interferon-γ (IFN-γ) and tumour necrosis factor-α (TNF-α), and reduce tissue rejections by inhibiting toll-like receptor 2 (TLR2)/1 and ensure the survival of insulin-producing MIN6 cells under inflammatory stress. Moreover, histological examination of the constructs post 4 weeks since their subcutaneous implantation in mice to investigate the tissue response to these constructs in vivo, did not show infiltration of multinucleated giant cells or granulocyte invasion underscoring the biocompatibility of the constructs. This study offers a better engraftment strategy for implanted islets in the treatment of type 1 diabetes.
Gelatin
Another polymer which has extensively been researched as a scaffolding biomaterial for tissue engineering applications is gelatin. It is obtained by the partial hydrolysis of collagen which is abundantly present in the ECM of living tissues [138]. Gelatin is essentially demonstrated thermoresponsive aqueous behaviour at temperature above 30℃ whereas it shows sol-to-gel transition at temperatures below 30℃ making it a suitable scaffold for various bioprinting applications [139]. Additionally, gelatin is biodegradable and biocompatible, with additional properties such as low immunogenicity. Moreover, gelatin is composed of different amino acids, particularly the biologically active RGD tripeptide sequence which promotes cell adhesion by interacting with integrins expressed on cell membranes. Therefore, the addition of gelatin can favor the adhesion, proliferation, and differentiation of beta cells in bioprinted constructs. Several research groups have demonstrated the efficacy of gelatin and its derivatives in encapsulating islets. Considering collagen is an essential component of the basement membrane of ECM in the adult human pancreas, gelatin has an advantage over other polymers in terms of providing the cells with a microenvironment close to their native one [140]. Mechanical strength is one of the most crucial properties demanded by pancreatic tissue engineering. This prerequisite is not met by gelatin alone. As a result, numerous gelatin-polymer mixtures have been investigated.
Another very important consideration while bioprinting structures for islet encapsulation is to maintain optimum porosity within the constructs to enable diffusion of nutrients. It is widely considered that in contrast to conventional bulk hydrogels, islets encapsulated within a porous bioplotted structure could have superior oxygen, glucose, and insulin exchange from surroundings due to a higher surface-to-volume ratio. The proof of concept for bioplotting cells within bioprinted constructs was reported by Marchioli et al. [136] who encapsulated INS1E β-cell line, primary human and mouse islets within an alginate/gelatin bioink. The resulting bioink had printable viscosity and thus could be used for printing macroporous self-standing constructs. Additionally, there were no detrimental effects of the printing process nor cytotoxic effect of material on the cells as validated by their high viability (91%) after 3 weeks. Nonetheless, the metabolic activity of cells encapsulated cells in the hydrogel decreased. The findings of the study revealed that the deterioration of functionality of encapsulated islets is not due to the bioplotting process, but rather results from reduced glucose diffusion through such a viscous hydrogel, such that even a macroporous structure like the one fabricated here cannot restore nutrition transport to reverse this loss of metabolic activity. This study perfectly highlights the inverse relation between the viscosity of material and bioplotting process where a lower material viscosity is beneficial for nutrient diffusion but not printable, while a printable material having higher viscosity creates a barrier for nutrient diffusion and hypoxic conditions affecting cell activity.
Previously in order to fabricate perfusable vascular constructs, gelatin methacryloyl (GelMA), a well-accepted bioink component, has also been used to enhance the printability of alginate/poly (-ethylene glycol)-tetra-acrylate (PEGTA) blended bioink to develop hollow perfusable constructs via coaxial bioprinting [141]. Following this trajectory, in a more recent study Liu et al. [142] co-printed islets, endothelial progenitor cells, and Tregs by adding GelMA to alginate bioink formulations to increase their printability, and stability, and to create an environment that supports transplanted islets in order to maximize islet survival (Figure 5). Scalable core-shell macroporous constructs for islet encapsulation were developed using a coaxial 3D bioprinter. The GelMA/alginate bioink formulations demonstrated shear-thinning behaviour, besides systematically improving to maintain structural stability and cell viability after the bioprinting process. Insulin secretion assessed under low and high glucose settings to assess the islet function following printing, revealed comparable insulin secretion levels in both printed and free islets, suggesting that the method of islet encapsulation and coaxial printing had no negative impact on their survival. Nevertheless, this novel study was the first of its kind to addressing issues related to re-vascularization and immunoisolation of islets via the fabrication of core-shell structures. The coaxial printed construct may shield the encapsulated islets in the core while simultaneously delivering various supportive cells into the shell owing to precise control over the positioning of multiple cells.
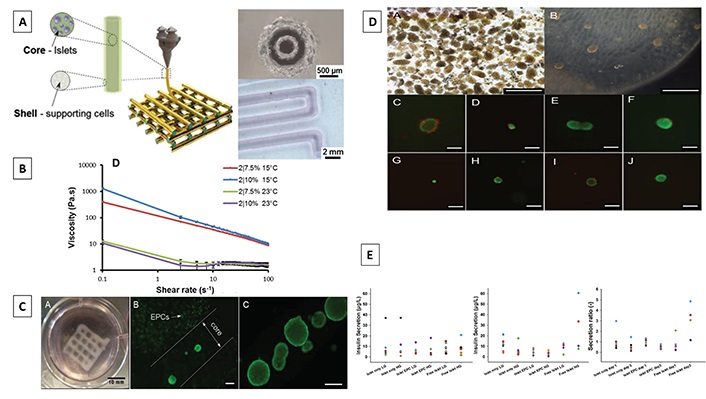
Coaxial 3D printed alginate/GelMA scaffolds for islet encapsulation. (A) Schematic representation of the coaxial printing approach carried out to fabricate vascularized bioartificial pancreatic constructs (left), microscope image of coaxial structure of printing nozzle (top right), and bright field image of coaxial strand printed with blue/red dye to visualize core-shell structure (bottom right). Scale bars depict 500 μm (top right), and 2 mm (bottom right); (B) shear thinning behaviour of different formulations of alginate/GelMA bioink. Data are presented in terms of mean ± SD (n = 3); (C) a coaxial printed construct with encapsulated islets and EPCs (left), printed islets with EPC (middle), and islets in core (right). Scale bars represent 200 μm in (B) and (C); (D) mouse pancreatic islets directly after isolation (top left) and immediately after encapsulation in alginate/GelMA (top right); live and dead staining of islets 24 h after encapsulation in alginate/GelMA scaffolds (middle and bottom row). Fluorescein diacetate/propium iodide was used to stain live (green) and dead (red) cells respectively. Scale bars represent 500 μm in (top row), and 200 μm in (middle and bottom row); (E) total secreted insulin after GSIS was measured at day 1 (left) and day 3 (middle) after printing, at low glucose (LG) and high glucose (HG) condition; and secretion ratio of insulin (right) per sample. GSIS: glucose-stimulated insulin secretion; SD: standard deviation; EPCs: endothelial progenitor cells
Note. Adapted with permission from “Development of a coaxial 3D printing platform for biofabrication of implantable islet-containing constructs,” by Liu X, Carter SD, Renes MJ, Kim J, Rojas-Canales DM, Penko D, et al. Adv Healthc Mater. 2019;8:e1801181 (https://onlinelibrary.wiley.com/doi/10.1002/adhm.201801181). © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
pdECM
High post-transplant islet loss appears to be caused by multiple factors, and is a major bottleneck towards their use for therapeutic applications [143]. One direct result of separation from the donor pancreas is the loss of connection between the islets and ECM molecules as well as the disintegration of the extensive vascular network that connects the islets during cell isolation protocols [144]. In addition, the loss of the thick microvasculature is responsible for impairments preventing the pancreatic islets from quickly detecting variations in blood sugar and responding by secreting insulin. These impairments include efficient oxygenation, nutrition delivery, and metabolic waste elimination [145]. Even while transplanted islets can endure for up to several days in an avascular environment, prolonged delays in tissue vascularization cause necrosis and loss of endocrine function [144]. As a result, it is believed that recapitulation of the native pdECM microenvironment, and a thick vascular network are essential for raising the likelihood of successful therapeutic results following islet transplantation procedures. The ECM in native tissue exhibits greater structural, chemical, biological, and mechanical complexity than these materials do [146]. For islets to function better, microenvironmental factors such as interactions between islets and ECM are crucial signals. Collagen, glycosaminoglycans (GAGs), and glycoproteins are some of the several ECM components that can be recreated using decellularized ECM (dECM). For instance, primary islets with peripheral ECMs still present (such as laminin, type I, III, IV, V, and VI collagen, and fibronectin) show lower levels of apoptosis and better insulin sensitivity, suggesting that tissue-specific cell-matrix interactions are crucial for improving the ability of these organoids to function similarly to native tissue [147]. Likely so, organs created through 3D bioprinting should be customized using particular tissue-specific biological inks. This can be done by 3D printing pancreatic islets using a specialized pdECM. Decellularized scaffolds mimic in vivo microenvironment for replanted cells by maintaining the general structure of natural ECM and inherent growth factors [148]. The pdECM hydrogels show improved and better function as derivatives of pancreatic decellularization. According to the concentration of cells and the requirements of the graft, different types of cells can be added to pdECM hydrogels, allowing for modulation of the hydrogel size and pdECM concentration [149]. Therefore, the selection of bioink is crucial in order to accurately recreate the target tissue’s microenvironment in the 3D-engineered tissue construct.
Tissue-specific dECM bioinks are useful for fostering stem cell differentiation and proliferation [150]. Because of its tissue-specific composition and architecture, dECM can act as a cell-favorable habitat in comparison to synthetic polymers [151]. The substantial retention of important components in the dECM should therefore require serious consideration of the decellularization process. In this regard, Kim et al. [152] used pdECM to create a native microenvironment for developing 3D pancreatic tissues that could be transplanted. The primary islets cultured in pdECM bioinks demonstrated excellent viability, enhanced insulin secretion, and functionality. Additionally, co-culture with HUVECs significantly prevented apoptosis of encapsulated islets in the center via the prevention of hypoxia arising from lack of vascularization. The viability of pancreatic islets printed in pdECM hydrogel was comparable to that of the control group comprising of non-printed islets in 3D culture.
Recently, Hwang et al. [153] aimed to create a novel engineering approach for the fabrication of a hybrid encapsulation system that encapsulates aggregates that resemble pancreatic islets using a multi-head bioprinting system and is composed of an assembled macroporous polymer capsule construct and nanoporous pdECM hydrogel. The outer part (macroporous PCL capsule) was fabricated with an interpenetrating architecture, allowing insulin-producing β-cells encapsulated within the hybrid scaffold to maintain their cellular behaviour, such as viability, cell proliferation, and insulin secretion. The inner part (nanoporous dECM hydrogel) composed of 3D biofabricated pancreatic islet-like aggregates was placed into the macroporous polymer capsule. With regard to M1 macrophage polarization which is indicative of inflammation, the developed hybrid encapsulation system demonstrated biocompatibility both in vitro and in vivo. Additionally, each component of the macroporous polymer capsule contained a porous membrane with a sub-micron size enabling the diffusion of nutrients and oxygen to the cells. Additionally, the macroporous polymer capsule offered retrievability and mechanical protection. In conclusion, these findings showed that the 3D bioprinting method makes it easier to create a hybrid islet encapsulation system using materials resembling native microenvironment of tissue may enhance clinical outcomes by promoting the structural maturation and functional advancement of cells.
Another recent study by Idaszek et al. [154] reported development of porous vascularized pancreas grafts via a microfluidic 3D bioprinting platform coupled with a co-axial needle system, using two different tissue-specific bioink compositions: an alginate/pECM bioink encapsulating pancreatic islets, and an alginate/fibrinogen bioink encapsulating HUVECs to develop pancreatic and vascular structures respectively. The resultant bioinks despite having varying rheological properties, were 3D-printed with high shape fidelity and viability thereby promoting endocrine function as reported by insulin secretion in response to glucose stimulation. Additionally, the ability of HUVECs to promote vascularization was confirmed by the formation of intercellular junctions and vessel-like structures by CD31-positive immunostained cells. Overall, the presented findings represented a significant advancement both in the field of biofabrication of vascularized pancreatic tissues and the applicability of multi-material/multi-cellular 3D bioprinting for engineering complex tissues like the pancreas.
Hyaluronic acid
Among the many bioinks currently being studied, hyaluronic acid, and its derivatives stand out because of their physiological relevance, cytocompatibility, shear-thinning capabilities, and ability to be tailored to specific characteristics through chemical modifications. The primary component of ECM and a natural polysaccharide known as hyaluronic acid is employed extensively in regenerative medicine and tissue engineering [155, 156]. It is essentially a non-sulphated disaccharide polymer composed of alternating N-acetylglucosamine and D-glucuronic acid disaccharide units linked by β-1,3 and β-1,4 glycosidic bonds [157]. It is biocompatible and biodegradable, making it completely safe bioprinting applications. According to earlier research, hyaluronic acid added to alginate for islet transplantation improves encapsulated cells’ survival rates [158] while also reducing the body’s immunological inflammatory response [159]. In the realm of tissue engineering, hyaluronic acid has been increasingly utilized as a scaffolding biopolymer. Islet cells that were encapsulated in hyaluronic acid and alginate matrices have reportedly shown high viability and insulin secretion [160]. Additionally, hyaluronic acid-alginate hybrid microcapsules have been demonstrated by Cañibano-Hernández et al. [158] to decrease apoptosis and boost β-cell survival. There is growing evidence available now which suggests that hyaluronic acid maybe a more suitable polymer for encapsulating islets in comparison to alginate which is most often used for cell encapsulation due to lesser immunogenicity.
Hyaluronic acid in its pure form, cannot be utilised as a material in bioink development since it lacks printability and is unstable due to its high-water absorption. Hyaluronic acid methacrylate (HAMA), obtained by the methacrylation process of hyaluronic acid can undergo quick gelation via photo crosslinking with lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) in the presence of ultraviolet (UV) radiation [161]. HAMA, a novel hydrogel ink for 3D printing, demonstrates quick photosensitivity, rapid gelation, and stable hydrogel performance. HAMA is additionally employed in the creation of biological organoids [162]. As a result, the mixture of HAMA and pdECM could serve as a source of bioink for 3D printing for making islet organoids. Recently, Wang et al. [163] fabricated an islet organoid via 3D printing using a hybrid bioink combining the mechanical properties of HAMA and tissue specificity of pdECM to simulate the in vivo pancreatic microenvironment (Figure 6). The developed HAMA/pdECM hydrogels were reportedly able to regulate islet adhesion, and morphology in vitro through expression of E-cadherin, N-cadherin, and fibronectin, which aids in islet function and activity through the ras-related C3 botulinum toxin substrate 1 (Rac1)/Rho-associated protein kinase (ROCK)/myosin light chain kinase (MLCK) signalling pathway. Consequently, a significant increase in the expression of pancreatic marker genes such as insulin (Ins2) and glucagon (Gcg) were reported via qPCR assay. The HAMA/pdECM hydrogels were consequently used for 3D-printing pancreatic islet organoids via a stereolithographic process, with reportedly high viability of islets observed with HAMA/pdECM thereby underscoring the cytocompatible nature of the material. Similar observations were reported during immunofluorescence analysis which indicated higher insulin and glucagon expressions of islets in the 3D-printed HAMA/pdECM hydrogels. The organoids were moreover able to respond appropriately to glucose stimulation via insulin release and were considered for implantation within diabetic mouse. The 3D printed islet organoids demonstrated excellent robustness in that they were able to retain their functionality even after 12 weeks of implantation, and reportedly restored blood glucose levels within a normal range (6.5 mM/mL) within 60 min of food intake. Additionally, the HAMA/pdECM hydrogels were found to significantly promote re-vascularization in vivo after 90 days of implantation as seen through high density of positive staining for CD31. All these findings demonstrated mimicking the native functionality of islets by the 3D printed HAMA/pdECM islet organoids.
3D-printed HAMA/pdECM pancreatic islet organoids. (A) digital camera micrographs of HAMA/pdECM hydrogels prepared using different concentrations of pdECM; (B) Western blot analysis to detect the protein expressions of E-cadherin, N-cadherin, β-catenin, Rac1, ROCK, and MLCK from HAMA/pdECM hydrogels; (C) quantitative PCR test evaluating the expression of the Ins2, and Gcg in the islets encapsulated within the hydrogels (n = 3); (D) live- and dead-cell staining of pancreatic organoids to detect the effect of 3D printing on the activity of islet organoids (Scale bar = 50 μm); (E) immunostaining micrographs of HAMA/pdECM islet organoids detecting the effect of 3D printing on islet function, including insulin (red) and glucagon (green) (scale bar = 50 μm); (F) glucose-stimulated insulin-release curve from the control, HAMA, and HAMA/pdECM pancreatic islet organoids; (G) blood glucose level in diabetic mice receiving transplantation (n = 6); (H) representative CD31 immunostained images on the surface and cavities of HAMA and HAMA/pdECM islets organoids. The blood vessels are stained red, and the nuclei are stained blue (scale bar = 200 µm). ‘S’ represents surrounding host tissue, ‘I’ represents the implanted hydrogel
Note. Adapted with permission from “Hyaluronic acid methacrylate/pancreatic extracellular matrix as a potential 3D printing bioink for constructing islet organoids,” by Wang D, Guo Y, Zhu J, Liu F, Xue Y, Huang Y, et al. Acta Biomater. 2022;165:86–101. (https://linkinghub.elsevier.com/retrieve/pii/S1742706122003750). © 2022 Acta Materialia Inc.
PLA
Compared with natural polymers, most synthetic polymers have super mechanical properties, and 3D printed structures can be maintained in vivo for a long time. PLA, is a widely used synthetic polymer in biomedical applications that is biocompatible, has high elasticity and mechanical strength suited for subcutaneous implantation, thus employed to promote the bio-integration of the encapsulating system [164]. Lactic acid is chiral in nature, making PLA hydrophobic and having poor cell adhesion properties. Surface treatment using plasma increases the low surface free energy of various materials and provides a solvent-free method for altering the wettability, surface chemistry and surface roughness of polymers, which enhances the cell survival and proliferation [165, 166]. The surface-free energy of PLA is also increased by plasma activation, generating a wide range of functional groups on the surface, including polar groups, which significantly alter wettability and have a favourable impact on material-cell interactions. Some studies demonstrated that plasma treatment significantly enhanced the surface’s hydrophilicity and decreased the contact angle, both of which were stable for 30 days in phosphate buffered saline (PBS) for over 30 days [167].
By optimizing the parameters of a low-cost 3D printer Song et al. developed a macroporous PLA scaffold that houses stem cell derived β-cell clusters within a degradable fibrin gel structure [168]. The diameter of cell clusters was calculated using a finite element model of cellular oxygen diffusion consumption in order to prevent severe hypoxia prior to vascularization in bioprinted constructs. Insulin was produced in response to a glucose injection following implantation of the constructs in mice, with the transplanted constructs maintaining their structural stability for 12 weeks following which they were retrievable. An important finding of the study was the non-responsiveness of the bioprinted grafts containing large cell clusters. Mice receiving implantation of these bioprinted constructs with big clusters reportedly had detectable amounts of human insulin which didn’t respond to glucose injection. Similarly, serum samples from mice receiving small clustered constructs showed the presence of human insulin. However, an approximately 2.50-fold increase in human insulin was induced by the glucose injection, showing that the transplanted grafts were functional and glucose-responsive. This strategy, as opposed to pure cell encapsulation methods, could be used as a foundation for sophisticated 3D printing strategies for diabetes cell replacement therapy.
For instance, Farina et al. [169], in their study, demonstrated that subcutaneous implantation of a functionalized, 3D-printed cell encapsulated system results in appropriate and rapid graft vascularization. PLA is used to develop an entirely novel implantable 3D printed device that will accommodate a pancreatic islets graft. Vascularization was enhanced by dispensing proangiogenic substances, such as vascular endothelial growth factor (VEGF), which is additionally known to improve islet viability and function [170]. Islets encased in this device could be protected from acute hypoxia and retain their function, resulting in insulin secretion over several months within the device. In order to meet changing physiological needs, the device’s transcutaneous refillability provides opportunities for cell augmentation without surgical extraction and re-implantation. Additionally, the reservoir structure allows for the possibility of retrieving the graft, which is crucial for designed cells made from stem cells that are experiencing malignant or other undesirable alterations.
PCL
PCL has become an increasingly popular choice among various types of biopolymers because of its multiple benefits such as biodegradability, high strength, and biocompatibility. PCL is a semi-crystalline thermoplastic polyester approved by the FDA that is manufactured by cationic or anionic ring-opening polymerization of ε-caprolactone at high temperatures [171]. Moreover, the potential for modification and functionalization with additional substrates like growth factors, and the development of a suitable scaffold design within a scalable process have convinced researchers to encapsulate cells within PCL scaffolds. Precise and complicated geometries could be created via 3D printing of PCL when additional sacrificial support materials are used. In essence, clinical islet transplantation incorporates the separation of islets from surrounding exocrine tissue in a donor via enzymatic digestion and mechanical disruption, accompanied by density centrifugation to purify the exocrine tissue [172]. Due to enzymatic degradation, pancreatic islet cells may often lose their interaction with the surrounding ECM. As a result, the natural microvasculature is disrupted causing cells to lose functionality. Although alternate transplant sites have been frequently proposed to address this challenge, the longevity of extra-hepatically transplanted islets is critically dependent on rapid re-vascularization following engraftment.
In line with a prior study by Marchioli et al. [173], developed a new 3D scaffold platform that could actively promote vascularization thereby having potential applications in extra-hepatic islet transplantation (Figure 7). Briefly, the authors prepared 3D ring-shaped PCL scaffolds surrounding an alginate hydrogel core for islet encapsulation. The heparinized surface of the PCL scaffold could bind VEGF to the alginate-encapsulated islets electrostatically, thereby improving vascularization in vivo and promoting cell adhesion because of decreased hydrophobic characteristics, greater protein binding capacity, and altered surface topography. When compared to the untreated PCL scaffold, heparin immobilization could increase VEGF retention in the scaffold by up to 3.6-fold. VEGF immobilized on the construct improved angiogenesis in close vicinity and on the surface of the scaffolds in a chicken chorioallantoic membrane (CAM) model. Moreover, islets encapsulated within the alginate core in these functionalized scaffolds showed a rounded morphology, and could be easily stained with DTZ confirming a positive response to glucose stimuli via insulin secretion, with results comparable to free-floating islets after 7 days. Overall, the aforementioned marks the development of a suitable scaffold that has the possibility of promoting rapid vascularization and endocrine function of encapsulated islets.
3D printed PCL/alginate ring-shaped scaffolds functionalized with VEGF for islets encapsulation. (A) The hybrid scaffold concept is depicted schematically. Briefly, the islets were encapsulated in the structure’s interior using alginate hydrogel and crosslinked using CaCl2. On the other hand, 3D plotted PCL rings adjacent to the alginate core were covalently functionalized using heparin to bind VEGF and safeguard it from degradation (left). PCL and PCL/heparin plotted scaffolds stained with Azure II (right); (B) SEM micrographs at different magnifications of the bare PCL scaffolds (top row) and of the heparinized constructs (bottom row) where the change in surface topography can be observed; (C) CAM assay using PCL and heparin-coated PCL scaffolds with three different VEGF concentrations. A 200 ng VEGF load resulted in leaking vessels on PCL scaffolds, whereas a higher VEGF concentration appeared to inhibit vessel formation. On heparin-coated scaffolds, 200 ng load VEGF appeared to induce the greatest number of blood vessels with normal morphology (arrows indicate vascularization); (D) the functional behavior of islets at day 1 (top) and day 7 (middle) when embedded within PCL/alginate hybrid scaffolds coated with heparin and functionalized with VEGF. Islets encapsulated in the alginate in the central core of the PCL/alginate scaffolds showing a round morphology and confirming the presence of insulin by staining with DTZ after 1 day of culture (bottom). SEM: scanning electron microscopy
Note. Adapted with permission from “Hybrid polycaprolactone/alginate scaffolds functionalized with VEGF to promote de novo vessel formation for the transplantation of islets of Langerhans,” by Marchioli G, Luca AD, de Koning E, Engelse M, Van Blitterswijk CA, Karperien M, et al. Adv Healthc Mater. 2016;5:1606–16 (https://onlinelibrary.wiley.com/doi/10.1002/adhm.201600058). © 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Growth factors
Beyond encapsulating cells within biopolymer matrices in the bioprinted constructs, cells could be signaled to migrate, proliferate, and differentiate in an engineered scaffold using bioactive substances such as growth factors. Growth factors are water-soluble physiologic polypeptides that have a powerful influence on many cellular activities and signaling pathways [174]. Hence, supplementation of growth factors into biomedical structures can stimulate cellular activity required for tissue regeneration inside an implant system. For instance, the significance of growth factors such as fibroblast growth factors (FGF) for the expansion of the early pancreatic rudiment [175] and later pancreatic development [176] has been demonstrated in embryonic model systems. It has been demonstrated that particular growth factors, like transforming growth factors, insulin and insulin-like growth factors, and VEGFs are involved in the proliferation and differentiation of pancreatic cells that secrete insulin and glucagon [177]. Some growth factors required for the differentiation and maturation of pancreatic cells are listed below in (Table 2).
Essential growth factors for 3D bioprinting pancreatic islets
| Sl. No. | Growth factors | Functions | References |
|---|---|---|---|
| 1. | Insulin-like growth factor (IGF) |
| [178–180] |
| 2. | VEGF |
| [181, 182] |
| 3. | FGF |
| [183] |
| 4. | EGF |
| [184, 185] |
| 5. | Keratinocyte growth factor (KGF) |
| [185–187] |
| 6. | Hepatocyte growth factor (HGF) |
| [188, 189] |
| 7. | Transforming growth factor (TGF) |
| [185] |
Sl. No.: serial number; bFGF: basic FGF; hESC: human epithelial stem cells
Strategies to recapitulate pancreatic function in bioprinted tissue
3D bioprinting is a highly novel and promising technology for precisely positioning biomaterials in conjunction with living cells, with the objective of fabricating tissue analogs. Despite its fascinating potential, the functional recapitulation of pancreatic tissue is a challenging endeavour. Keeping into consideration that the fabricated constructs are able to recapitulate functional aspects of their native counterparts, substantial effort has been placed on improving cell proliferation and in vitro maturation of bioprinted constructs. Towards achieving this goal in the context of a bioartificial pancreas, researchers have sought to delve deeper into more fundamental aspects to improve the in vitro functionality of encapsulated islets in terms of glucose sensing and insulin secretion.
Pancreatic islets are assemblages of hormone-secreting endocrine cells that rely on complex cell-to-cell interaction mechanisms to function properly. When islet cells become dispersed, they exhibit a strong potential to reaggregate, and once clustered, they produce more insulin in response to glucose [190]. This architecture of islets has been found to be significant for their functions, implying that individually dispersed islets or simple aggregates may have comparatively inferior functions. When undamaged islets are stimulated with glucose, cell-to-cell contact is essential for the commencement of insulin release [191]. The use of high cell density in bioinks appears to be the most obvious approach for allowing close proximity between islets within a given space, hence favoring aggregation. An alternative to this is the use of more sophisticated bioprinting technologies which allow for printing spheroids, which are self-assembled 3D aggregates of cells capable of mimicking tissue function realistically [192].
The introduction of any foreign object into the body triggers an inflammatory and fibrotic process known as the foreign body reaction [193]. When a foreign object is implanted into a tissue, immune system cells migrate towards it and attempt to eliminate it. 3D bioprinting uses bioinks that contain biomaterials and cells that have the potential to activate immunological responses inside the body. Foreign body responses against implanted bioprinted constructs can damage the biomaterial scaffolds, and can interfere with the long-term survivability of the encapsulated cells, thereby limiting engraftment. In such circumstances, immunoisolation methods are used to prevent the non-self-antigens on the graft (bioprinted construct) from coming into touch with the host immune system [194]. Strategies to achieve immunoisolation have focussed on modifying the biomaterial surface encapsulating the cells such that it can counter immune responses. Towards this end Hu et al. [87] developed a tri-component bioink containing pectin for encapsulating pancreatic islets. The pectin incorporated bioprinted constructs supported cell viability, and shielded the cells from inflammatory stress due to foreign body reaction by inhibiting the activation of TLRs. Advances in bioprinting technologies have come up with newer approaches like coaxial bioprinting, which allows for printing core-shell structures where an exterior shell structure which safeguards the inner islet containing core from immune response [142]. As already mentioned, such core-shell structures can also accommodate various supportive cells in the shell, to improve cellular crosstalk between supportive cells and islets.
The inability to appropriately vascularize tissues in vitro or in vivo currently limits tissue engineering approaches such as bioprinting. Nutrient perfusion and mass transport constraints, particularly oxygen diffusion resulting in hypoxic conditions, limit construct development to dimensions lesser than clinically applicable and limit in vivo integration due to cell death. A recent study by Kim et al. [195], reported the production of free radicals in hypoxic conditions, which negatively affected islet cells in terms of decreased insulin production, macrophage cell infiltration, and reduced viability due to apoptosis. Additionally, since insulin secretion in islets necessitates a great deal of mitochondrial respiration, islets consume a lot of oxygen and nutrients relative to their proportion in the pancreas [196]. In native tissue, the microvasculature of islets comprises a highly specialized network of arterioles, capillaries, and venules. Endocrine cells receive nourishment by blood due to the large number and fenestration of capillaries, allowing for a quick exchange of nutrients and hormones, rapid monitoring of blood glucose variations, and the accompanying counterbalancing hormone (insulin) outflow [197]. Presently, 3D bioprinting-based technologies employ vascularization to address hypoxia issues [198]. Induction of vasculature within bioprinted constructs is typically achieved using co-culture systems involving supportive cells such as endothelial cells (e.g., HUVECs), which over time organize to form primitive microvascular networks through the process of vasculogenesis, and eventually help the neotissue achieve biomimetic functionality [199]. An advancement to this strategy is the incorporation of additional supportive cells like fibroblasts, and MSCs to foster functional vascularization and engraftment of islets in a physiological and biomimetic fashion. MSCs and fibroblasts are known to help the endothelial cells during the vascularization process [200]. Additionally, MSCs also promote angiogenesis by remodeling the ECM, secreting pro-angiogenic factors like VEGF, angiopoietin 1 (Ang 1), Ang 2, and stabilizing vasculature [201]. However, controlling the vascularization process and the proper organization of microvascular networks within bioprinted constructs remain a challenging task to date [202].
All endocrine cells collaborate to form a sophisticated paracrine network that guarantees adequate blood glucose management. Furthermore, interactions between endocrine cells and other cellular microenvironments, such as vascular cells, and innate immune cells, are required for proper endocrine network formation and function [197]. Extrusion-based 3D bioprinting is the ideal technique for fabricating anatomically appropriate living cell-laden 3D structures in vitro due to the ability to achieve spatiotemporal directional positioning of distinct cells by using multiple extrusion nozzles. Although the application of redrawing the original cellular environment with multiple pancreatic cell types has not yet been documented, we highlight future studies in this area.
Conclusions
Commenting on the present scenario, immense progress has been achieved in the field of islet encapsulation and 3D bioprinting towards developing bioartificial pancreas. A clinically relevant endocrine pancreas fabricated through bioprinting has the potential to revolutionize the treatment of T1DM by providing a viable alternative to conventional insulin therapy. Millions of people with T1DM could see a significant improvement in their quality of life if functional, implantable endocrine pancreatic tissue that can produce insulin in response to changes in blood glucose levels could be developed by 3D bioprinting. In this review, our focus has been to primarily highlight the bioprinting process of endocrine pancreatic tissues, with special emphasis on the various biomaterials, cell types, growth factors, and strategies to recapitulate pancreatic islet function. The review of existing literature presented in this article highlights the possibility of eradicating pre-existing bottlenecks in islet encapsulation techniques through bioprinting. In this review, we have introduced bioprinting as a technology highlighting its stages, and types. Without doubt, EBB stands out among the other bioprinting modalities, in terms of versatility, usage of high cell densities, and printing multiple cell types through multiple nozzles attached to a single printhead. Because of the higher density of extrusion-based printing, there is an obstacle to delivering oxygen and nourishment to the cells within the matrix. In order to resolve this issue, porous scaffolds, interconnected channels, and induction of vascular networks have been developed. Additionally, keeping into consideration the need for immunoisolation, and incorporation of supportive cells to rule out hypoxia through formation of vascular networks, coaxial extrusion bioprinting is suggested to be best suited for bioprinting pancreatic islets. The choice of cell source is a very crucial aspect in the case of bioprinting applications. Ideally, it is preferable to fabricate implantable bioartificial pancreases from autologous cells or stem cell-derived pancreatic cells for custom or personalized pancreas engineering [203]. A substantial amount of the immune-mediated rejection of bioprinted pancreas is capable of being overcome using autologous cells. A significant number of live cells with no immunogenicity ensures organ-level replacement and functional response.
In the majority of the bioprinted pancreatic islets reviewed in this article, it has been demonstrated that islet cells can retain high activity and release insulin within bioprinted constructs. These studies give sufficient reason to consider 3D bioprinted pancreas to become the mainstream treatment for T1DM in the near future. However, there exist certain unresolved challenges to be addressed in order to obtain a completely implantable bioartificial pancreas. For instance, bioprinted constructs fabricated using pure natural polymers and ECMs are mostly devoid of adequate mechanical properties, and hence maintaining their original form before the cells mature into mature tissues is difficult [203]. On the other hand, bioprinted constructs made of pure synthetic polymers are difficult to load with cells and bioactive agents. To produce a functional bioartificial pancreas with appropriate mechanical strengths and biological activities, material scientists must search for new polymers, or design new biomaterial blends for bioink development [204]. Bioink development has numerous well reported biopolymers at its disposal which could be investigated for their suitability as pancreatic bioinks. One such polymer is silk fibroin which despite belonging to natural origin has excellent mechanical properties, is FDA approved, and has been extensively used as a bioink material for different tissues [115]. In the realm of pancreatic tissue engineering, silk hydrogels have reportedly been used by Davis et al. where the islets could maintain their viability and functionality [205]. Moreover, the beneficial role of silk on pancreatic islets was reported by Do et al. [206] where oral ingestion of silk fibroin hydrolysates promoted the maintenance of pancreatic β-islet integrity, and improved insulin secretion supporting the use of silk for developing bioinks. In the future, silk-based bioinks should be developed and characterized to validate these independent findings on their possible beneficial role on islet cells. Another important direction for pancreatic bioink development could be the use of antioxidant polymers like carrageenan, and chitosan for developing bioinks with antioxidant properties [207, 208]. Such polymers in addition to exerting cytocompatible effects on the cells, may allow to combat oxidative stress encountered by the implanted biomaterial as a part of surgery performed during implantation [209]. This would also improve the post-implantation recovery and enhance the chances of engraftment.
Thus the future direction of the 3D bioprinting of endocrine pancreatic tissue mainly includes (1) enhancing bioprinting methods to allow for the more precise and high-resolution printing of complex structures, (2) finding new biomaterials that will promote the development and differentiation of islets and enhance their in vivo performance, (3) investigating the use of bioreactors to create a controlled environment for the bioprinted tissues, enhancing cell survival and function, and (4) conducting well planned clinical trials to evaluate the safety and efficacy of bioprinted endocrine pancreatic tissue in humans in patients with T1DM. Overall, endocrine pancreatic bioprinting has advanced significantly despite existing challenges, and ongoing research shows great promise for the future. To overcome the remaining challenges and create an effective treatment for T1DM, continued collaboration between specialists in bioprinting technology, stem cell research, gene editing, and clinical practice is probably essential.
Abbreviations
| 3D: | three dimensional |
| ADSCs: | adipose-derived stem cells |
| ASCs: | adult stem cells |
| CAD: | computer-assisted designing |
| dECM: | decellularized extracellular matrix |
| DM: | diabetes mellitus |
| DTZ: | dithizone |
| EBB: | extrusion-based bioprinting |
| ECM: | extracellular matrix |
| FDA: | Food Drug Administration |
| FGF: | fibroblast growth factor |
| GelMA: | gelatin methacryloyl |
| HAMA: | hyaluronic acid methacrylate |
| hiPSCs: | human induced pluripotent stem cells |
| HUVECs: | human umbilical vein-derived endothelial cells |
| iPSCs: | induced pluripotent stem cells |
| LAB: | laser-assisted bioprinting |
| MSCs: | mesenchymal stem cells |
| PCL: | polycaprolactone |
| pdECM: | pancreatic-derived extracellular matrix |
| PLA: | polylactic acid |
| PP-cell: | pancreatic protein cell |
| RGD: | arginine-glycine-aspartic acid |
| T1DM: | type 1 diabetes mellitus |
| Tregs: | regulatory T-cells |
| VEGF: | vascular endothelial growth factor |
Declarations
Author contributions
AG and AS : Writing—original draft, Formal analysis, Investigation, Writing—review & editing. AM: Conceptualization, Writing—original draft, Formal analysis, Investigation, Writing—review & editing, Validation, Writing—review & editing, Supervision. All authors have read and agreed to the submitted version of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Data from the present manuscript is available from the corresponding author upon reasonable request abhikmallick1988@gmail.com.
Funding
Not applicable.
Copyright
© The Author(s) 2023.