Abstract
Background:
The naturally occurring metabolite lactate has traditionally been considered as a waste product of anaerobic metabolism whose production was confined to hypoxic states. However, more evidence supports that lactate is a preferred oxidative substrate for the myocardium when present in high plasma levels. Small-scale clinical studies have found that infusion of exogenous hypertonic sodium lactate (HSL) increases cardiac output and stabilizes the haemodynamic profile. More comprehensive studies investigating haemodynamic or cardiovascular effects of HSL have been conducted using different animal models of disease.
Methods:
We performed a broad systematic search of electronic databases PubMed and Embase to identify animal studies in which haemodynamic or cardiovascular effects of HSL were reported. A total of 135 studies were identified. Nineteen studies were included in this review. Different disease models were included including sepsis (n = 4 studies), cardiac arrest (n = 3 studies), myocardial infarction (n = 2 studies), haemorrhagic shock (n = 3 studies) while studies on healthy hearts (n = 4 studies) were included. Also, three studies investigating the cardioprotective and cardiometabolic roles of HSL were included.
Results:
The review explains several beneficial haemodynamic effects of HSL infusion during a variety of disease states including increased cardiac output, increased microcirculation, and decreased systemic inflammation. Only a few studies identified the negative effects of HSL infusion. This paper suggests that high doses of lactate serve as an important cardiac fuel during crises and can also function as a pH buffer. However, the review revealed significant flaws in the reporting quality of the majority of studies.
Discussion:
HSL infusion holds promise as a potential treatment fluid for a variety of diseases. Future translational studies should focus on enhancing reproducibility, study design, and study reporting.
Keywords
Metabolism, lactate, hypertonic, inflammation, cardiovascular, animal studiesIntroduction
The traditional view of lactate as a by-product of anaerobic metabolism during hypoxic state has led to its reputation as a worthless or potentially harmful substance [1]. Often blood levels of lactate are used to evaluate the severity of illness in diseases like heart failure, acute myocardial infarction (MI), after cardiac arrest, and sepsis [2–5]. However, the disease states leading to lactate accumulation, rather than lactate per se, are to blame. In fact, myocardial lactate deprivation is associated with earlier death and worsened outcomes during endotoxic and haemorrhagic shock [6, 7]. Also, current research compellingly indicates that lactate production is not exclusive to an-aerobic conditions but also occurs during fully aerobic circumstances, even in the resting state [8–10] suggesting that lactate production is a fundamental physiological process that occurs in both health and disease.
The healthy heart exhibits metabolic versatility, capable of utilizing various substrates such as free fatty acids (FFA), glucose, ketone bodies, lactate, and amino acids, with a preference for FFA under normal conditions [11, 12]. Recent studies have highlighted lactate as a promising fuel source that can significantly enhance cardiac function in patients with heart failure [13] and during exercise [14]. Furthermore, increased myocardial lactate consumption during heart failure is associated with increased stroke volume and improved left ventricle efficiency [15].
Due to the tenuous negative repute of lactate, research on the topic has not been considered very appealing, though highly relevant. Therefore, only a limited number of studies have been conducted to determine its precise haemodynamic and cardiovascular effects and its potential as a treatment option. Historically, small-scale clinical trials involving human patients have been conducted on patients after cardiac arrest, during atrioventricular block [16], and in patients with cardiac arrhythmias during cardiac surgery [17]. More recently studies have been done on healthy volunteers [18], patients with acute heart failure (AHF) [13], patients with cardiogenic- or septic shock [19], and patients during or after cardiac surgery [20–22]. All studies report the beneficial effects of hypertonic lactate infusion on cardiac haemodynamics. Though fascinating, these pioneering studies are relatively few and vary in terms of quality as many have limited sample sizes, lack of randomization, or lack of blinding. More comprehensive translational trials utilizing different animal models and disease scenarios have been carried out. Also, the number of animal studies exceeds those in human, which enables the possibility to compare the results of the animal studies in the same diseases, across different diseases and species.
This systematically performed review aimed to examine the existing evidence from animal studies to elucidate the cardiovascular and haemodynamic effects of exogenous hypertonic lactate infusion. The objective was to clarify the current understanding and shed light on knowledge gaps to enhance translatability of the animal studies.
Materials and methods
Data sources and search strategy
A systematic review was performed focusing on study designs of hypertonic lactate infusion in animal models. The PRISMA 2020 guidelines [23] were followed wherever possible.
We did a systematic search of the electronic PubMed and Embase databases for animal studies involving the infusion of hypertonic exogenous lactate reporting haemodynamic or cardiovascular outcomes. Forward and backward citation searches were performed for the included trials.
We conducted a comprehensive search with high sensitivity using terms that encompassed our primary concepts of animal models receiving exogenous lactate infusion with a goal of raising blood levels of lactate (Figure 1). Only original studies that measured and reported on haemodynamic or cardiovascular endpoints and administered exogenous sodium lactate were included. We excluded all reviews, protocols, abstracts, studies not published in peer-reviewed journals, studies not in English, and studies that did not involve animal models. This review only included animal models as one of the main aims of this review is to enhance the reproducibility and credibility of animal studies in the future. Relevant studies involving human subjects are included in the discussion.

Search string. *: truncation to find multiple endings of the root word. The original sensitive search string was used to search for articles on PubMed and Embase. Upwards and downwards citation searches were carried out on identified hits
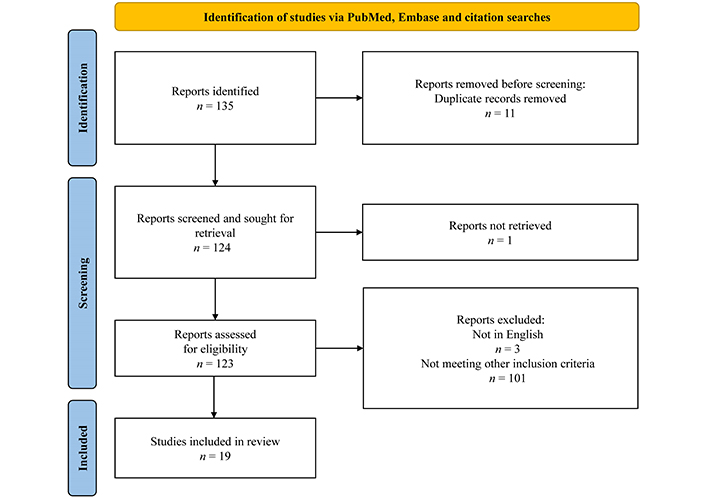
A total of 135 studies were identified. Eleven duplicates were removed. One study was not found. Three studies not in English were removed. One hundred and one studies were removed due to not meeting the inclusion criteria. The vast majority of these studies were removed due to the sensitivity of the search string being very high which led to identification of many studies not investigating the effects of lactate on hemodynamic or cardiovascular function. Nineteen studies were included (see PRISMA diagram in Figure 2). A search for reporting of randomization and blinding was conducted in all included studies by using search terms (random) and (blind).
Results
In the succeeding sections, the following abbreviations for treatment options will be used: hypertonic sodium lactate (HSL), hypertonic sodium bicarbonate (HSB), hypertonic saline (HS), and normotonic saline (NS).
Disease models
Lactate infusion have been tested in a variety of disease settings in animal models (Table 1). These include four studies of lactate infusion during septic- or endotoxic shock [24–27], three studies of lactate infusion as resuscitation fluid during or after cardiac arrest [28–30], two studies of lactate infusion in myocardial infarction [31, 32], and three studies involving haemorrhagic shock [33–35]. One study was a drug discovery test involving an AHF model [36] and two studies investigated lactate in treatment of cardiotoxic events [37, 38]. This review furthermore included four studies involving HSL for healthy hearts [39–41] and one study involving physiological levels of lactate in an isolated healthy heart [42].
Study characteristics and major findings
| Author & year | Country | Animal | Randomization | Blinding | Lactate dosage | Major findings | Ref |
|---|---|---|---|---|---|---|---|
| Sepsis (n = 4) | |||||||
| Duburcq et al.2014 | France | Pigs | Yes | Not reported | 5 mmol/(kg h) for 4.5 hours |
| [25] |
| Su et al.2016 | Belgium | Sheep | Yes | Partly blinded* | 1.5 mmol/kg bolus for 15 minutes followed by continuous 0.5 mmol/(kg h) for 2 hours |
| [27] |
| Duburcq et al.2017 | France | Pigs | Yes | No | 5 mmol/(kg h) for 4.5 hours |
| [26] |
| Besnier et al.2020 | France | Rats | Yes | Not reported | 2.5 mmol/(kg h) for 18 hours |
| [24] |
| Cardiac arrest (n = 3) | |||||||
| Miclescu et al.2007 | Sweden | Pigs | Yes | Not reported | 1.89 mmol/(kg h) for 50 minutes |
| [30] |
| Stevic et al.2022 | France | Rabbits | Yes | Yes | 5 mmol/(kg h) for 2 hours |
| [28] |
| Annoni et al.2023 | Belgium | Pigs | Yes | No | 10 mmol/kg bolus followed by 1.8 mmol/(kg h) for 12 hours |
| [29] |
| Healthy hearts (n = 4) | |||||||
| Hørsdal et al.2024 | Denmark | Pigs | Yes | Yes | 5 mmol/(kg h) for 2 hours |
| [39] |
| Onay-Besikci2007 | Turkey | Rats | Not reported | Not reported | 0.1 mol/L for duration of experiment in perfusion fluid |
| [42] |
| Barthelmes et al.2010 | Switzerland | Pigs | Yes | Not reported | Different infusion rates for 20 minutes each: 2.0, 4.0, 6.0, 8.0 mmol/(kg h) |
| [40] |
| Bellet et al.1957 | USA | Dogs | No | No | A variety of infusion rates between 21 mmol/(kg h) to 210 mmol/(kg h) |
| [41] |
| Myocardial infarction (n = 2) | |||||||
| Zhang et al.2021 | China | Mice | Not reported | Not reported | One-time 22.44 mmol/kg intraperitoneal bolus per day |
| [31] |
| de Groot et al.1993 | Netherlands | Rats | No | No | 0.5 mmol/L in perfusate for 45 minutes |
| [32] |
| Haemorrhagic shock (n = 3) | |||||||
| Kline et al.2000 | USA | Rats | Not reported | Not reported | 8.0 mmol/L in perfusate for 60 minutes |
| [33] |
| Rocha e Silva et al.1993 | Brasil | Dogs | No | No | 6 mmol/kg bolus |
| [34] |
| Schmoker et al.1991 | USA | Pigs | Yes | No | 2 mmol/kg bolus followed by continuous infusion to restore baseline MAP |
| [35] |
| Cardioprotection (n = 2) | |||||||
| Haege et al.2021 | USA | Zebrafish | Not relevant† | Not relevant† | N/A |
| [36] |
| Bellet et al.1959 | USA | Dogs | No | No | 4 mmol/kg bolus for 4–5 minutes |
| [37, 38] |
* blinding of operators was not done. Blood flow video data underwent blinded analyses; † study was a drug discovery study. All animals were treated equally; CO: cardiac output; ECG: electrocardiographic; EDP: end diatolic pressure; HSL: hypertonic sodium lactate; LVEF: left ventricular ejection fraction; MAP: mean arterial blood pressure; SvO2: mixed venous saturation
Study results
Effects of lactate on healthy hearts (n = 4 studies)
In 2024 our study group, Hørsdal et al. [39] demonstrated the haemodynamic effects of HSL infusion in healthy pigs. The study was a randomized, blinded cross-over study where HSL was administered for 2 hours and compared with euvolemic and tonicity matched control saline. Cardiac output (CO) increased during HSL infusion due to vasorelaxation and increased heart rate. Furthermore, mixed venous saturation (SvO2), left ventricular ejection fraction (LVEF), and cardiac efficiency were increased while mean arterial blood pressure (MAP) remained unaltered. Veno-arterial CO2 difference [P(v-a)CO2] was decreased during HSL infusion. Finally, HSL resulted in metabolic alkalosis.
In 2010 Barthelmes et al. [40] addressed the haemodynamic effects of HSL infusion in healthy pigs during central venous and portal vein administration of HSL. Pigs were randomly allocated to receive HSL in either a central vein, the portal vein, or an NS infusion. Animals were studied for 80 minutes of infusion and 60 minutes post-infusion. They found that pigs allocated to one of the HSL groups had a significantly greater CO than did the NS group while heart rate was unaltered in the infusion periods. HSL was administered at rates of 1, 2, 3, and 4 mL/(kg h) for 20 minutes at each step. NS infusions were at the same rates. They found that HSL groups had an increased CO than did the NS group. They also found increased blood flow of hepatic and femoral arteries during HSL infusion. Interestingly, they also found that HSL groups had a lower arterial- and mixed venous oxygen saturation in the hour following the infusions.
In 1957 Bellet et al. [41] examined the effects of lactate infusion in healthy dogs. The group found that infusion of 1 molar HSL at a rate of 0.5 mL/(kg min) increased the CO without altering MAP. If, however, infused at doses of more than 0.5 mL/(kg min) the group found depressed MAP, decreased force of myocardial contraction and electrocardiographic (ECG) changes consistent with infusion induced hypopotassemia.
In 2007 Onay-Besikci [42] demonstrated that physiological levels of 1 mM lactate in the perfusion solution on healthy rat hearts, had no effect on cardiac parameters including heart rate, CO, MAP, and aortic output.
Cardiovascular effects during sepsis (n = 4 studies)
In 2020 Besnier et al. [24] used rats and induced shock by fecal peritonitis. They included four study groups. One control group and three sepsis groups. The sepsis groups received either HSL, NS, or HSB with equivalent amounts of sodium and osmoles as HSL. Four of six of the first rats in HSB died, hence this group was not completed. The groups were observed for 18 hours. They found beneficial haemodynamic and cardiovascular effects during HSL as compared with NS including increased CO, left ventricular fractional shortening, and dP/dtmax. They found no effect on end systolic pressure volume relationship therefore the dP/dtmax increase was likely due to preload- or afterload alterations. The group also found improved mesenteric microcirculation measured using laser speckle contrast imaging on the gut, lowered end-diastolic pressure-volume relationship, and increased blood levels of the ketone body 3-OHB. Furthermore, they found HSL to decrease capillary leakage and reduce inflammation as they found a lower level of IL-1, TNF-alpha, and VEGF-A. Finally, they found that HSL infusion led to a negative fluid balance.
In two independent studies, Duburcq et al. [25, 26] used pigs as animal model and utilized intravenous injection of E. coli endotoxin to induce hypodynamic shock. In 2014 they included three groups (n = 5 in each) where one received HSL, one control group received NS and one group received HSB with equal amounts of osmoles and sodium as well as the same alkalizing effect as the HSL group. In 2017 Duburcq et al. [26] repeated the study with the addition of 39 g of glucose to the infusions in the HSB and NS groups to infuse an equivalent energy supply as the SL group. In both studies, the pigs were observed for 5 hours. In both studies, Duburcq et al. [25, 26] found that HSL was superior to HSB and NS regarding haemodynamic effects. In 2014 the group found that CO was less impaired by the endotoxic shock after 5 hours of observation in the HSL group compared with NS (p = 0.01) while they found no significant difference between HSL and HSB groups. They also found that MAP was significantly better in the HSL group compared with HSB and NS. They also found improved microvascular reactivity with lower P(v-a)CO2 difference in the HSL group compared with both HSB and NS. In 2017 the group, again, found significantly improved CO in the HSL group, as well as improved MAP. SvO2 and P(v-a)CO2 were also improved in the HSL group. The authors found this to complement the finding of higher CO and improved peripheral perfusion. While the pH gradually decreased in the NS group, HSL and HSB resulted in metabolic alkalosis. Finally, the group found that HSL led to a negative fluid balance measured as fluid infusion volume vs urine output.
In 2016 Su et al. [27] used sheep and induced hyperdynamic shock by fecal peritonitis. They included an HSL group, an HS group, an NS group, and a Ringer’s lactate (RL) group in their study. The sheep were observed until spontaneous death. The group found decreased CO in the HSL group compared with the HS group after 12 and 16 hours. Also, they found more severe microcirculatory alterations in the HSL group. The same was true for the renal blood flow, again indicating low CO and end-organ hypoperfusion. Finally, the survival time was shorter in the HSL group vs HS or RL group.
Cardiovascular effects during cardiac arrest (n = 3 studies)
Miclescu et al. [30] used a pig model of cardiac arrest with 12 minutes of cardiac arrest and 8 minutes of cardiopulmonary resuscitation (CPR). Cardiac arrest was induced using alternating transthoracic current. Animals were studied for 4 hours. They tested three different treatment options during CPR, all including methylene blue (MB). The three options were MB in NS, MB in saline dextran, and MB in HSL in randomized order. Pigs receiving MB in HSL had lower levels of troponin I and CK-MB than pigs receiving MB in NS indicating less myocardial damage. Also, the pulmonary capillary wedge pressure (PCWP) was increased in the MB in NS group after return of spontaneous circulation (ROSC). HSL furthermore had alkalizing effects. They concluded that MB in HSL had similar effects as MB in saline dextran and that MB in HSL had better cardioprotective effects than MB in NS. They found no difference in survival or markers of cerebral injury.
Stevic et al. [28] used a cardiac arrest model on rabbits in 2020. The study was randomized and blinded. Cardiac arrest was induced by the withdrawal of mechanical ventilation in paralyzed animals. After 12.5 minutes of untreated arrest CPR was initiated until ROSC. The animals were studied for 2 hours. After stabilization rabbits would receive either continuous infusion of NS or HSL for 120 minutes. After 120 minutes HSL had significantly improved MAP, ventricular shortening fraction, and CO as compared with NS. Also, the LV end-diastolic pressure, the filling pressure, was lower in the HSL group (p = 0.003). The diuresis was higher in the MSL group (p = 0.014) and resulted in a neutral fluid balance as well as a higher pH. Finally, they found that HSL had a neuroprotective effect. They found no difference in survival.
Annoni et al. [29] used a pacing wire to induce cardiac arrest in a pig model in 2023. The study was randomized. After 10 minutes of cardiac arrest chest compressions were provided for 5 minutes before the first bi-phasic electric countershock. Epinephrine was administered after 1 minute of cardiac arrest. Epinephrine and electric shock were provided every minute if ROSC was not present after the first round. Resuscitated animals were observed for 12 hours. The group randomized the pigs to receive either bolus NS followed by continuous infusion of balanced crystalloid, bolus HSL followed by continuous infusion of HSL, or bolus NS followed by continuous infusion of HSL. The group found that significantly lower doses of norepinephrine were required to maintain a MAP > 65 mmHg in both HSL groups compared with the NS group. The group also found that circulating levels of troponin I were lower in the HSL groups compared with the NS group. They also found lower levels of markers of cerebral damage in the HSL groups. They found no difference regarding CO, PCWP, or other haemodynamic parameters. They found no difference in survival. Infusion of HSL also mitigated the severe metabolic acidosis during cardiac arrest.
Cardiovascular effects during and after myocardial infarction (n = 2 study)
In 2019 Zhang et al. [31] permanently ligated the left anterior descending (LAD) artery inducing a myocardial infarction in a mouse model. The mice were injected with HSL or NS one day after surgery and consecutively for 14 days. After 14 days the mice were investigated with echocardiography. They found that HSL attenuated post-myocardial infarction dysfunction by improving ejection fraction (EF) and ventricular fraction shortening. They concluded that HSL could improve cardiac function after myocardial infarction. They also found significantly less myocardial fibrosis upon examination. Finally, they found HSL to enhance the expression of STAT-3 which is known to play multiple protective roles in the heart [43] possibly leading to anti-inflammatory polarization of macrophages. The study does not report on randomization or blinding.
A 1993 study by de Groot et al. [32] investigated the effects of exogenous lactate, glucose, and pyruvate in the perfusion fluid of isolated rat hearts after 15, 30, and 45 minutes of ischemia on coronary flow. The study found that while the flow of the inner layers of the LV was reduced in lactate perfused hearts the flow in the outer layers was improved. Also, the relative flow in the right ventricle was increased.
Effects of lactate in haemorrhagic shock (n = 3 studies)
In 2000 Kline et al. [33] used a model of haemorrhagic shock utilizing rats that were bled out in order to investigate the effects of lactate in the perfusion solution. They randomized the hearts to either of the following groups of solution: i) glucose only, ii) glucose and palmitate, iii) glucose and palmitate and lactate, iv) glucose and high palmitate, or v) glucose and high palmitate and lactate. The main cardiovascular findings by the group were that the addition of 8.0 mM lactate significantly improved cardiac work in shocked hearts and increased cardiac efficiency. They concluded that lactate could improve rather than deteriorate cardiac energy metabolism after haemorrhagic shock.
A 1993 study by Rocha e Silva et al. [34] tested hypertonic sodium dextran against other hypertonic sodium dextran solutions: hypertonic sodium acetate dextran, and HSL-dextran (HSLD) in a dog model of uninterrupted arterial bleeding. 80 dogs of approximately 17 kg were included. The study found that HSLD increased CO measured by thermodilution and MAP compared to all other groups. HSLD also reversed shock-induced acidosis. The group concluded that an increase in CO and MAP, however, led to a higher cumulated blood loss.
In 1991 Schmoker et al. [35] studied early and late effects of HSL in a pig model of haemorrhagic shock after bleeding the animals to a MAP of 50 mmHg. Animals were randomized to receive either HSL or RL as a bolus followed by continuous infusion. The group found no difference in CO or MAP during a 24-hour follow-up period, but cerebral oxygen delivery increased in the HSL group as intracranial pressure (ICP) was reduced. The group concluded that the ICP reduction was due to cerebral dehydration due to the hyperosmolar composition of the HSL fluid ultimately leading to the increased cerebral blood flow and oxygen delivery.
Lactate as a cardioprotective treatment (n = 3 studies)
A 2021 drug discovery study in a zebrafish model of AHF conducted by Haege et al. [36] identified lactate as a cardiac protectant. They found that lactate inhibited inflammation and cardiac hypertrophy. AHF was induced by aristolochic acid which led to human-like disease with cardiac hypertrophy, severed cardiac fibers, loss of endocardium, and gradual weakening and subsequent cessation of cardiac contractility. The study was designed to identify natural products that were beneficial to AHF, hence the authors tested nearly seventy herbal crude extracts. They identified the compound A2-4-2-4 which inhibited inflammation and cardiac hypertrophy by reducing MAPK signaling activity as well as COX-2 gene expression. MAPK cascades are known to modulate hypertrophic responses to pressure overload [44, 45] as well as the promotion of inflammatory cytokines. COX-2 is known to play a key role in the development of cardiovascular disease [46]. Following chemical analyses found that A2-4-2-4 was almost pure lactate. Subsequently, the authors tested pure sodium lactate and found attenuation of AHF and inflammation as well as cardiac hypertrophy. The authors concluded that lactate could serve as a cardiac protectant and a new therapeutic agent for AHF.
In 1959 Bellet et al. [38] investigated the effects of 1 molar sodium lactate infusion in dogs during quinidine intoxication. Dogs were sorted into one of two groups: a control group receiving nothing but continuous quinidine infusion until death (n = 7) or a group receiving quinidine in the same manner and treated with one molar HSL during various stages of quinidine intoxication (n = 12). Cardiotoxic effects of quinidine, which manifested as hypotension and ECG alterations. These effects were reversed in the HSL group during the early stages of intoxication with increased blood pressure and reduction of ECG changes. The group described several possible mechanisms. First, infusion of a hypertonic fluid leads to the expansion of extracellular space and thereby the concentration of quinidine was reduced. Second, infusion of HSL leads to alkalosis which shifts potassium ions from extracellular space into the cells reducing cardiotoxic hyperkaliemia. Also, the group found an increase in blood pressure which could be the cause of the increase in CO and coronary flow, the expansion of plasma and blood volume, or a combination of these. Finally, the group argued that lactate per se may act as a myocardial substrate in the Krebs cycle as a readily oxidizable fuel. In more advanced stages of quinidine intoxication, the group found that HSL was not able to reverse the cardiotoxic effects.
Later in 1959, the same group used a similar setup to examine the effects of HSL on cardiotoxic effects of procaine amide intoxication [37]. The group found that HSL was able to diminish the cardiotoxicity of procaine amide even during later stages of intoxication. Again, the group described several possible mechanisms. First, a decrease of procaine amide in the same way as observed in the quinidine study. Second, improvement of intoxication-induced acidosis and hyperkaliemia as HSL leads to alkalosis. Finally, a direct metabolic effect of lactate.
Discussion
Lactate as a therapeutic agent––overview
Energy source
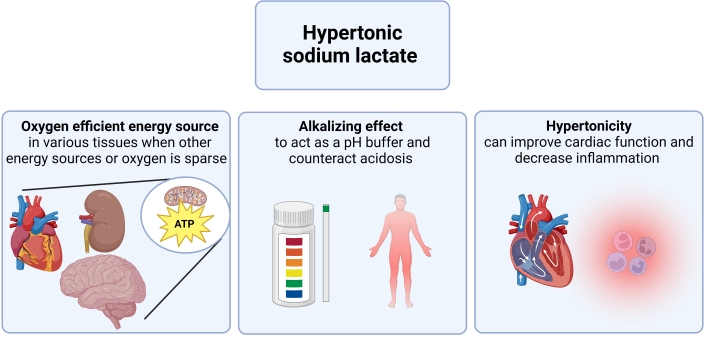
Lactate serves as an alternative energy source (Figure 3). Recent research developments have transformed the perception of lactate from merely a metabolic byproduct to an active participant in glucose metabolism. Lactate is produced and metabolized even in the resting state in healthy tissues in the brain, heart, skeletal muscles, and kidneys [10]. During heavy workloads or under stress situations such as ischemia, the heart can switch its energy source to available substrates and lactate becomes particularly important in this context as it is readily available for utilization in the myocardium [10, 47]. Furthermore, the ATP yield per O2 molecule of lactate exceeds that of FFA, the heart’s preferred fuel [48, 49]. Also, the utilization of lactate infusion has been suggested to provide beneficial cerebral metabolic effects due to its role as an energy substrate after traumatic brain injury [50]. Hence, the use of the lactate molecule per se as a direct substrate for ATP generation may play a role in maintaining a stable hemodynamic and cardiovascular profile in terms of CO, decreased myocardial damage, and neuroprotection.
HSL may have different mechanisms in different situations. Lactate per se is an oxygen-efficient fuel that is readily oxidizable in many fuels. Hence, lactate can be an important alternative energy source during hypoxic state or when other fuels are sparse. The alkalizing effect of lactate enables it to work as a pH buffer able to revert acidosis, which can be present during hypoxia i.e., sepsis or cardiac arrest. Hypertonicity of HSL can also contribute to increased cardiac function, increased sensitivity to endogenous vasopressors, and decreased inflammation.
Alkalizing effects
When administered as sodium-lactate, lactate has an alkalizing effect which was also demonstrated in several of the included studies in this review. This potential to act as a buffer and modify pH may play a role in its therapeutic effects, as it can counteract the acidosis often seen in states of hypoxia or ischemia. This property might explain some of the findings in e.g., septic shock or following cardiac arrest as discussed in this review. Also, alkalosis per se may improve cardiac function, and could maybe explain some of the findings in the mentioned studies [51]. Notably, the studies by Duburcq et al. [25, 26] found significantly different hemodynamic effects when comparing lactate to another alkalizing agent, favoring lactate while the study by Besnier et al. [24] had to exclude the bicarbonate group due to excess mortality. Whether this is translatable to other diseases has not been investigated.
Hypertonicity
The hypertonic nature of lactate solutions has shown benefits, such as in CO enhancement following cardiac surgery [21, 22]. Hypertonicity of saline fluids has been linked to enhanced contractile force of the heart as well as anti-inflammation [52]. Therefore, the observed anti-inflammatory effects of lactate-fluids in this review may in part be explained by the tonicity rather than lactate as a fuel. It is worth noticing that the osmotic effects of hypertonic fluids including HSL may help manage cell swelling and edema, e.g., during states with increased ICP[53]. Notably, when compared with other hypertonic fluids i.e., in cardiac arrest and sepsis, HSL still proved superior. Hence, despite the hypertonicity of HSL having undeniable hemodynamic effects, this alone does not seem to explain the findings.
Effects of lactate in different diseases
Haemodynamic effects of lactate in healthy animals
Hørsdal et al. [39] demonstrated that CO increased in a large animal model due to increased heart rate. Also, systemic vascular resistance was decreased indicating vasorelaxation. While MAP was unaltered HSL infusion demonstrated other beneficial effects involving increased LVEF due to afterload decrease and increased cardiac efficiency. The authors conclude that this might be due to lactate becoming an increasingly utilized substrate for oxidative phosphorylation with increased plasma levels. The findings in the study are somewhat in contradiction to the results by Barthelmes et al. [40] who also utilized healthy pigs as an experimental model as this study group found the increase in CO to be mediated by increased stroke volume rather than heart rate.
Interestingly, Barthelmes et al. [40] also find self-contradictory results. On one hand, the authors found that CO increased during HSL infusion. On the other hand, the authors claim that HSL infusion led to decreased SvO2, a surrogate measure of perfusion and CO [54]. There may, however, be an explanation for this observation. Although the study protocol by Barthelmes et al. specified that pigs should be ventilated to maintain a PaO2 level above 13.3 kPa, this was not uniformly followed across all groups. Only the NS group adhered to the ventilation protocol, while the HSL groups were allowed to reach PaO2 levels as low as 8.5 kPa, resulting in arterial oxygen saturation of 91%. Consequently, the decline in SvO2 may potentially be attributed to inadequate ventilation rather than reflecting changes in CO. Unfortunately, the authors did not provide detailed information on the specific ventilator settings, making it impossible to delve further into the ventilation differences between the groups. Supporting the theory that HSL infusion increases CO in healthy animals Bellet et al. [38, 41] found that HSL infused in non-toxic doses increased CO and did not compromise MAP. In a separate study involving healthy hearts, Onay-Besikci [42] found that the addition of physiological amounts of lactate had no effects on cardiac function in isolated rat hearts. These findings indicate the presence of a therapeutic interval in which lactate has a positive effect on the CO. Levels above or below this interval level either compromise cardiac function or have no effect whatsoever.
In conclusion, in recent times only two studies on healthy large animals have been conducted. Only one of these reported randomization and blinding. The findings in these studies are to some degree contradictory and future randomized blinded studies are needed to fully clarify the haemodynamic effects of HSL in the healthy heart. These studies should ensure proper maintenance of ventilator settings to guarantee adequate comparability among groups with respect to oxygenation parameters. Also, studies should try to determine the therapeutic interval levels at which lactate has beneficial effects on cardiac and cardiovascular function. Finally, there is room for improvement regarding the reporting quality. Onay-Besikci’s study lacked both reporting of randomization and blinding, while the study by Barthelmes et al. was randomized but lacked reported information on blinding. The study by Hørsdal et al. was randomized and blinded. The study by Bellet et al. also lacked the reporting quality of modern standards.
Use of lactate in sepsis treatment
Sepsis is associated with profound deficit in effective blood volume due to vasodilation and capillary leakage. Therefore, large amounts of intravenously infused fluids are often used aiming to restore volume status, CO, and organ perfusion. This however often happens at the expense of tissue oedema formation that may influence organ function [55–57]. Low volumes of hypertonic fluids could therefore have beneficial effects. However, the high concentrations of chloride may lead to kidney injury [58, 59]. The evidence from animal studies investigating the effects of exogenous HSL infusion for sepsis in the present review, though limited to four studies, indicates that HSL infusion could prevent tissue oedema or chloride-induced kidney injury as three studies found that HSL led to negative fluid balance and HSL contains less chloride than HS.
The fact that high lactate levels during sepsis could be beneficial is supported by data provided by a study from 2007 that used live rats undergoing endotoxic shock. The study reported that myocardial lactate deprivation was associated with decreased cardiovascular performance and energetics as well as early death [6].
The large animal studies done by Su et al. [27] and Duburcq et al. [25, 26] found somewhat opposing results. In the comparison of these studies, several factors should be considered. First, Su et al. used an ovine model while Duburcq et al. used pigs. This, however, should not have any influence on the results as both large animal models are capable of mimicking human physiology and thoracic anatomy [60–62]. Secondly, both groups used randomization for treatment allocation while only Su et al. reported blinding of data analysis. Blinding generally enhances the validity of the results. Thirdly, one remarkable difference between the studies was the amount of lactate infused. While Su et al. only infused 200 mmol during a 15-hour period, Duburcq et al. infused 450 mmol during a 4.5-hour period, a total of more than seven times more lactate infused. Therefore, the infused amount of lactate by Su et al. may be insufficient. Finally, Duburcq et al. argued that the HSL group in the study by Su et al. was under-resuscitated and received 30% less volume than the HS group. On the other hand, Su et al. claimed that Duburcq et al. 2014, used a less clinically relevant model with fixed-dose fluid resuscitation. Though there remained some discrepancies between study results, most studies found that HSL led to a beneficial haemodynamic profile with improved MAP, CO, and improved organ perfusion also indicated by increased diuresis. Still, it has not been clarified whether the beneficial effects of HSL during sepsis, are due to a direct effect of lactate as a metabolic substrate or an effect of the associated metabolic alkalosis or hypertonicity. Notably, infusion of HSB and HSL had similar effects on acid-base balance with metabolic alkalosis in the studies by Duburcq et al. Still however, in the studies by Duburcq et al. despite similar acid-base effects, and similar tonicity the hemodynamic effects of HSL compared to HSB were significantly enhanced including higher MAP and CO while the HSB group was excluded prematurely in the study by Besnier et al. [24] due to excess mortality.
Future studies are needed to fully clarify the cardiovascular and haemodynamic effects of sodium lactate infusion. Especially, the effects of different doses, infusion rates, and differences between dynamic- and fixe-dose fluid regimes of HSL infusion during sepsis should be examined. Also, the exact cardiovascular mechanism of action of lactate needs to be investigated. Furthermore, the quality of reporting should be improved. Though all four studies were randomized only two studies reported that they did not include blinding of operators while the remaining studies did not report on blinding.
Use of lactate in treatment of cardiac arrest
The cardiac arrest studies included in this review all find HSL infusion to result in a more beneficial haemodynamic profile if given during or after CPR compared with control. Small-scale clinical studies conducted in 1955 also found HSL to be beneficial in treatment of cardiac arrest [16]. However, the studies are not without limitations. First, while all studies were randomized only Stevic et al. [28] performed a blinded study. Annoni et al. [29] reported that blinding of operators was not feasible while analysis of electroencephalographic data was performed blinded. Miclescu et al. [30] did not report any blinding. Secondly, only Miclescu et al. used hypertonic fluid as a control fluid. Also, all of the used models of cardiac arrest are devoted to achieving the highest degree of ROSC, while still having a sufficient no-flow time to induce cardiac- and brain damage. Furthermore, the animals used were otherwise young and healthy and had no comorbidities. This contrasts with most cardiac arrest patients who are older and often display several comorbidities that can influence the cardiac arrest pathophysiology and treatment [63–65]. Therefore, many cardiac arrest models fail to replicate the severity of human cardiac arrest and are often of limited translatability to the clinical setting [66]. Additionally, the models used in the included studies only examine the animals for 2 to 12 hours, hence the longer-term effects of treatment with HSL during cardiac arrest on survival and organ damage have yet to be elucidated. In all studies, the administration of HSL had an alkalizing effect. A higher pH has been proposed to increase the vascular reactivity to endogenous vasopressors and ultimately increase the ROSC rate [67, 68]. Hence, it remains unclear whether the beneficial effects of HSL infusion, including the decreased need for vasopressors, is a result of lactate as a substrate, or rather the alkalizing effects.
In the future studies could benefit from a design that more closely mimic human cardiac arrest [69]. Also, the studies could use hypertonic control fluids to account for the increased tonicity of HSL and the thereby following haemodynamic effects which include increased CO [20, 70, 71]. Additionally, the optimal infusion regime and dose of HSL should be clarified. Furthermore, to shed light on the longer-lasting effects, the observation period of included animals should be extended. Also, studies comparing HSL with other drugs with alkalizing effects are warranted. Finally, reporting quality should be improved. Though, all three studies were randomized, only two of three studies reported on blinding. One of these two studies reported that the study design involved blinding.
Despite the limitations, the positive effects of HSL in these innovative studies cannot be ignored: improved MAP, improved CO, decreased markers of cardiac injury, decreased markers of brain injury, and less need for norepinephrine during resuscitation. Though these findings were not all reproduced across all studies, they are still of great interest and HSL should be further investigated as a resuscitation fluid.
Use of lactate during myocardial infarction
Only one study has investigated the effect of HSL treatment in a situation of in vivo MI. Hence, the need for more studies to ensure reproducibility appears obvious. Also, the included study did not use a control infusion of equal tonicity and therefore did not account for possible effects of hypertonicity. Despite these limitations, mouse models play pivotal roles in the research of MI as they express similarity in many genes with humans as well as overall anatomical similarities [72, 73]. Also at the chronic stage, the mouse heart remodels with systolic dysfunction as the human heart after MI [74]. Still, small animal models have some shortcomings compared with large animal models such as pigs or sheep. Large animal models share similarities in size, anatomy, and physiology of the human heart and thorax as well as a more complex immune system. Therefore, making predictions of human responses to therapies is more approachable if large animal models are used [60, 75]. In the future, in vivo studies should make sure to compare HSL treatment for MI against another hypertonic fluid to diminish the effects of hypertonicity. Also, future studies could benefit from using larger animal models to make the results more clinically relevant. Again, reporting quality should be improved. The included study reported neither randomization nor blinding.
Use of lactate during haemorrhagic shock
Though infusion of HSL led to higher cumulated blood loss in the study by Rocha e Silva et al. [34] this is most likely a side effect of the increased CO and MAP found by the group. Despite this, HSL infusion seems a beneficial treatment for haemorrhagic shock if proper volume replacement is being performed to account for increased blood loss due to several factors. First, restoration of circulation including improving MAP and CO is the most important thing during the acute management of haemorrhagic shock [76]. Maintenance of oxygen delivery is essential to prevent hypoxia, inflammation, and organ dysfunction [77]. Secondly, HSL could reverse shock-induced acidosis. Third, evidence suggests that lactate has beneficial metabolic effects including improved cardiac efficiency. While Kline et al. [33] found that HSL increased cardiac work and cardiac efficiency after haemorrhagic shock, in 2000 Barbee et al. [7] found that depletion of endogenous lactate reduced the cardiac efficiency in a similar model of isolated rat hearts after haemorrhagic shock. Studies in human patients with congestive heart failure found that increased myocardial lactate consumption was associated with lower myocardial oxygen consumption and increased stroke volume [15]. Hence, HSL infusion has several beneficial properties if administered during uncontrolled bleeding and haemorrhagic shock. However, the therapeutic priority must be to stop the bleeding to prevent lethal blood loss.
Lactate as a cardioprotective drug
In a drug discovery study conducted by Haege et al. [36], lactate was identified as a potential cardioprotective agent for AHF. The researchers discovered that lactate administration resulted in the downregulation of MAPK activity and COX-2 gene expression. As a result, they observed reduced cardiac hypertrophy and inflammation. In a separate study conducted by Besnier et al. [24], the infusion of HSL was found to decrease the levels of pro-inflammatory cytokines, including TNF-α. The findings from Haege et al. provide a possible explanation for the decreased cytokine levels observed by Besnier et al., as increased MAPK pathway activity is known to elevate the levels of pro-inflammatory cytokines, such as TNF-α [78]. TNF-α exerts as a pathogenic factor in cardiac fibrosis and heart failure associated with a large decrease in survival [79] and can upregulate COX-2 further increasing levels of inflammatory cytokines [80, 81]. During their study of HSL infusion during MI, Zhang et al. [31] found that HSL led to less myocardial fibrosis and enhanced expression of STAT-3. STAT-3 is known to play multiple protective roles in the heart and downregulate the production of pro-inflammatory TNF-α production by monocytes [82, 83]. Hence, the findings by Zhang et al. could provide another explanation for the findings of Besnier et al. Together the findings of the studies included in this review indicate that lactate could serve as a cardioprotective drug in multiple situations as MI or AHF by regulation of multiple cardioprotective and anti-inflammatory pathways.
The early studies by Bellet et al. [37, 38, 41] suggest that HSL per se has beneficial metabolic effects. This agrees with the findings already discussed in earlier sections of this review. The healthy heart favors FFA for ATP generation while the failing heart has a higher dependency on lactate and ketone oxidation [84]. Though the ATP yield per molecule is greater for FFA’s than for lactate the ATP yield per consumed oxygen molecule is greater for lactate than FFA [13, 47]. A 2014 clinical study found that HSL infusion increased CO in patients with AHF [13]. Together these findings indicate that lactate could prove a vital energy substrate in a situation of a starving myocardium e.g., heart failure.
Concluding remarks
The number of studies investigating the haemodynamic and cardiovascular effects of exogenous lactate continues to grow. However, the number of conducted studies remains limited, and their quality varies, particularly in terms of reporting randomization and blinding. Despite these varieties in reporting qualities, most evidence suggests that exogenous HSL could be an alternative treatment method in a variety of situations and disease states. Lactate has beneficial effects in myocardial crises such as shock situations, cardiac arrest, and myocardial infarction. Henceforth, the field of metabolic approaches to these situations remains highly relevant. Future studies should prioritize the development of high-quality randomized blinded translational studies with strong clinical translatability and comprehensive reporting. Also, future studies should emphasize the alkalizing and hypertonic properties of HSL to clarify whether the hemodynamic effects of HSL infusions are explained by these, or by the utilization of lactate as a fuel. This approach will enhance the quality of data, translational value, reproducibility and lead to novel insight into how we can utilize hypertonic lactate fluids in the future.
Abbreviations
| AHF: | acute heart failure |
| CPR: | cardiopulmonary resuscitation |
| CO: | cardiac output |
| ECG: | electrocardiographic |
| FFA: | free fatty acids |
| HS: | hypertonic saline |
| HSB: | hypertonic sodium bicarbonate |
| HSL: | hypertonic sodium lactate |
| HSLD: | hypertonic sodium lactate-dextran |
| ICP: | intracranial pressure |
| LVEF: | left ventricular ejection fraction |
| MAP: | mean arterial blood pressure |
| MB: | methylene blue |
| MI: | myocardial infarction |
| NS: | normotonic saline |
| P(v-a)CO2: | veno-arterial CO2 difference |
| RL: | Ringer’s lactate |
| ROSC: | return of spontaneous circulation |
| SvO2: | mixed venous saturation |
Declarations
Author contributions
OKH: Conceptualization, Methodology, Formal analysis, Visualization, Writing—original draft, Writing—review & editing.
Conflicts of interest
OKH has conducted experimental research involving treatment with lactate as an intervention. He has no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
The primary data for this systematic review were sourced online from databases listed in the methods. Referenced articles are accessible on PubMed and Embase databases. Additional supporting data are available from the corresponding author upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2024.