Abstract
Background:
Oral mesenchymal cells already have wide clinical applications based on their tissue regenerative abilities. The purpose of this study is to present the picture of scientific research on the application of mesenchymal cells.
Methods:
This review study presents data processing of selected articles on clinical application of oral mesenchymal cells as a future of technology with relatively reduced cost. As inclusion criteria, there are articles that evaluated the regenerative abilities of cells prior to their oral origin. The exclusion criteria are mainly laboratory procedural techniques of manipulation with mesenchymal cells. From 735 articles screened for retrieval, 148 articles were found. After application of exclusion criteria, it was reached in total, about 38 selected articles were analyzed based on combinations of keywords on the PubMed page. These articles were classified based on concrete positive results and evasive results of studies on the role, mechanism of action, and field of application of oral mesenchymal cells.
Results:
The selection ratio of mesenchymal cells of pulpal origin or of periodontium origin is based on the first type of cells. Regardless of the fact that the trend of their application is again in the oral cavity, in a smaller percentage they tend to be applied for tissue regeneration in other organs.
Discussion:
There is a lack of “in vivo” type studies. The trend of articles is about review-type studies on the field of application of oral mesenchymal cells. Articles, where the field of application of mesenchymal cells is beyond the oral cavity for the purpose of application in regenerative medicine, occupy a reduced percentage. There are significant differences between differentiating abilities depending on the source from which these cells are taken from the oral cavity. This ability can be modeled by using growth factors, cytokines, bioactive substances, or local anesthetics.
Keywords
Stem cells, mesenchymal, dental pulp, regeneration, periodontalIntroduction
The oral origin of mesenchymal cells causes them to be differentiated initially based on the tissue where they originate [1–4]. Pulpal or periodontal mesenchymal cells are understood to be separated depending on the type of dentition or the stages of their eruption [4–14]. The regenerative ability of these cells is based on the properties that these cells carry genetically, properties that affect tissue regeneration in the areas where they are applied. Among these anti-inflammatory properties and high biocompatibility are among the most important, especially in the process of tissue regeneration. the clinical success of their application [7]. The mini-invasive technique with its advantages and the use of tissues in not so visible small areas in space, but in comparison with the clinical results of the application of oral mesenchymal cells, are elements that, in comparison and from a comparative perspective, only lean towards positive advantages with indications of clinical application [14–25].
Mesenchymal cells combined with growth factors and biomaterials make a trio of elements, each of which has a special importance for the long-awaited process of tissue regeneration. Regenerative endodontics is the newest orientation of tissue regeneration in the oral cavity, where mesenchymal cells can mainly be applied orally [8, 12]. The therapeutic treatment of teeth can be a field where regenerative endodontics can be applied [13, 21, 25]. Regardless of the specific origin of the oral mesenchymal cells, they unite at one point in the regenerative ability, in the anti-inflammatory ability, and in the adaptive ability to the area where they are applied against the specific immune cells there [7, 17].
Materials and methods
The conducted study is of the systematic review type, with the aim of finding the orientation of scientific research for oral mesenchymal cells in tissue healing and regeneration in the host areas where they are applied. In the PubMed page, different combinations of keywords were applied with the aim of finding as many articles as possible that are included in this topic. The orientation of the electronic search was mainly dependent on the oral origin of the mesenchymal cells, such as pulpal or periodontal. The application of filters in the electronic search and the time limit of five years significantly reduce the number of articles collected during the initial stages of selection [1–45]. The date of the last electronic search was 12.04.2024. The re-check was carried out on 12.08.2024, but there were no articles added during this period. After the initial analysis of the selected articles and after distinguishing the articles that were not specifically included in the scope of the study, it was possible to keep 38 articles for further analysis. As inclusion criteria in the selection of articles, there were articles that evaluated the regenerative abilities of oral mesenchymal cells prior to their origin, pulpal or periodontal, in the host area where they were applied with the purpose of tissue regeneration. The exclusion criteria were mainly tissue laboratory tissue procedures [27, 33, 35] and procedural techniques of manipulation with oral mesenchymal cells [18, 32, 42, 45].
Results
Articles selected in accordance with the purpose of the study will be classified based on the information they convey, depending on the type of publication presented. The selection of articles and the way in which the final number of articles was reached is shown by the PRISMA flow diagram in Figure 1.
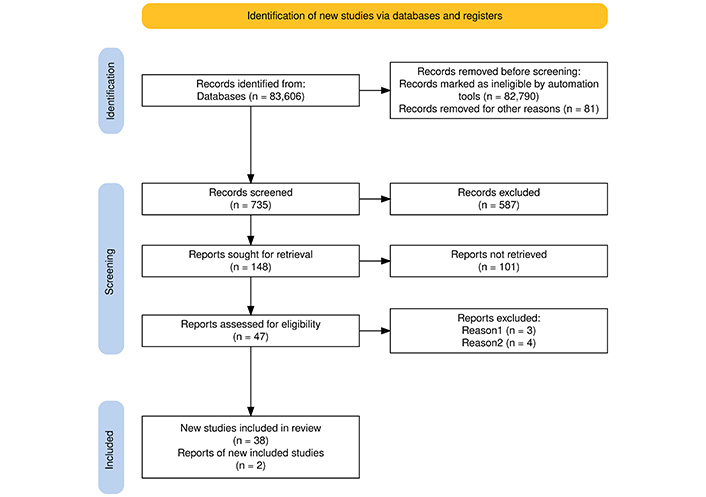
PRISMA flow diagram that shows the way of selecting articles for further analysis in support of the purpose of the article’s topic. Adapted from [46]. © 2021, The Author(s). CC BY 4.0
Table 1 analyzes only the articles with information about mesenchymal cells of pulpal origin. In this table, positive or negative results are shown again depending on the purpose of the study of the articles selected for analysis. Table 2 shows quantitatively the articles divided based on the purpose of the study of the article and the results obtained from this study, classified as concrete results and evasive results. The purpose of this table is to show in a quantitative way how many articles positively evaluate the application of mesenchymal cells of pulp origin and how many articles show evasive results about this application. In other tables, only articles containing information on mesenchymal cells of periodontal origin are analyzed, with concrete or effective results shown based on the purpose of the study.
Data on positive or negative results of articles about the purpose of studies where mesenchymal cells were applied
| Purpose of study | Concrete results | Evasive results |
|---|---|---|
| Role of cells | 0 article | Xie Z et al. 2021 [1]—Promising preclinical and clinical results on the tissue-directed engineering solution in clinical cases. |
| Mechanism of action | Zhang S et al. 2020 [17]—The produced exosomes promote migration, proliferation, and odontogenic differentiation. | 0 article |
| Field of application | Hernandez-Monjaraz B et al. 2020 [13]—Periodontal diseases are cured with clinical success by application to pulpal mesenchymal cells, as these reduce the effect of superoxide dismutase and IL-1 beta. | Yamada Y et al. 2020 [34]—Pulpal mesenchymal cells are used for bone regeneration, periodontitis, and regeneration of the pulp itself. |
| Li Y et al. 2019 [23]—Mesenchymal cells from inflamed pulps regenerate bone and repair periodontal defects, confirmed 3 months post-surgery. | 0 article | |
| Hernandez-Monjaraz B et al. 2018 [37]—Increase bone density and reduce periodontal pocket depth, promising for periodontal disease treatment. | 0 article | |
| Adding cellular effect | Adamička M et al. 2021 [9]—Non-steroidal anti-inflammatories and paracetamol increase the osgteogenic and chondrogenic effects of pulpal mesenchymal cells. | Divya TC et al. 2021 [36]—Aspirin modulates the differentiating effect of pulp mesenchymal cells. |
| Pupo YM et al. 2021 [21]—Collagen-hydroxyapatite networks increase stability, migrate proliferation of pulpal mesenchymal cells. | 0 article | |
| Yuan M et al. 2018 [22]—Aspirin increases the osteogenic effect. | 0 article | |
| Fageeh HN et al. 2021 [30]—The present periodontitis reduces the migratory, healing effect but not the osteogenic effect of pulpal mesenchymal cells. | 0 article | |
| Barbosa RM et al. 2023 [44]—Chitosan, xanthan, and hydroxyapatite membranes in proportion 1:1:2 increase the balance of mechanical and biological properties of pulpal mesenchymal cells. | 0 article | |
| Total | 0/1/3/5 | 1/0/1/1 |
Based on the concrete and evasive results obtained from the clinical application of mesenchymal cells of pulp origin, the orientation of the articles selected for analysis is determined
| Purpose of study | Concrete results | Evasive results |
|---|---|---|
| Role of cells | 0 article—0% | 1 article [1]—3% |
| Mechanism of action | 1 article [17]—3% | 0 article—0% |
| Field of application | 3 articles [13, 23, 37]—8% | 1 article [34]—3% |
| Adding cellular effect | 5 articles [9, 21, 22, 30, 44]—13% | 1 article [36]—3% |
| Total | 9 articles—24% | 3 articles—8% |
It should be noted that the percentages of the total number of articles are based on the total number of articles and not by adding the percentages according to the relevant columns of the table
In Table 3, only the articles with information about mesenchymal cells of both pulpal and periodontal origin are analyzed. In this table, again depending on the purpose of the study, concrete or effective results are shown based on the purpose of the study of the article with orientation about the role of mesenchymal cells of periodontal origin.
Data on concrete or effective results of articles about the purpose of studies where mesenchymal cells of periodontal origin have been applied, classified on the role of mesenchymal cells in their clinical application
| Purpose of study | Concrete results | Evasive results |
|---|---|---|
| Role of cells | Andrukhov O et al. 2019 [11]—Mesenchymal cells from inflamed dental tissues have compromised immunomodulatory abilities. | Galler KM et al. 2021 [2]—Different activation mechanisms of mesenchymal cells are highlighted, explaining the close interplay between inflammation and regeneration and inflammation and the potential for malignant transformation. |
| Costela-Ruiz VJ et al. 2022 [14]—Dental mesenchymal cells have better proliferation ratios, and those of gingival origin show higher clonogenicity. | Zhou LL et al. 2020 [6]—Oral mesenchymal cells have the ability to interact even in inflammatory microenvironments. | |
| Mercado-Rubio MD et al. 2021 [25]—Follicular and periodontal ligament mesenchymal cells express higher lipid-accumulating abilities than pulp cells when induced for adipogenic differentiation. | Chen Y et al. 2023 [8]—Epigenetic regulation means changing the level of expression and function of the gene without changing its sequence. | |
| Qu G et al. 2021 [26]—The highest osteogenic ability cells from the periodontal ligament, then those from the pulp, and the lowest those from the dental follicle. | Li B et al. 2021 [10]—Immunomodulatory functions enable the orchestration of the surrounding immune microenvironment. | |
| Total | 4 articles—11% | 4 articles—11% |
In Table 4, only the articles with information about mesenchymal cells of both pulpal and periodontal origin are analyzed. In this table, again depending on the purpose of the study, concrete or effective results are shown based on the purpose of the study of the article with orientation about the mechanism of action of mesenchymal cells of periodontal origin.
Based on the mechanism of action of mesenchymal cells, articles concerning mesenchymal cells of periodontal origin are presented, and their results are evaluated as either positive or evasive based on the analysis conducted
| Purpose of study | Concrete results | Evasive results |
|---|---|---|
| Mechanism of action | Hosoya A et al. 2020 [3]—Sonic hedgehog protein produced by epithelial mesenchymal cells with GLI1 receptor in mesenchymal cells serves for pulpal and periodontal regeneration. | 0 article |
| Mai Z et al. 2021 [4], Hua S et al. 2021 [5]—Exosomes from mesenchymal cells are actively involved in intercellular communication, anti-inflammation, osteogenesis, angiogenesis, immunomodulation, and promoting tumor cell apoptosis. | 0 article | |
| Zeb Khan S et al. 2021 [43]—The expression of cell proliferation markers of mesenchymal cells for the healing of periapical processes is confirmed. | 0 article | |
| Total | 4 articles—11% | 0 article—0% |
In Table 5, only the articles with information about mesenchymal cells of both pulpal and periodontal origin are analyzed. In this table, again depending on the purpose of the study, concrete or effective results are shown based on the purpose of the study of the article oriented around the field of application of mesenchymal cells of periodontal origin.
The data collected from the articles on the field of application of mesenchymal cells of periodontal origin
| Purpose of the study | Concrete results | Evasive results |
|---|---|---|
| Field of application | Li W et al. 2022 [20]—Mesenchymal cells from periapical lesions demonstrate potential use for dental pulp regeneration. | Wang D et al. 2019 [15]—Cells from the neural crest can be used for nerve repair as they have a reduced rejection ratio compared to others. |
| Gaubys A et al. 2018 [16]—Regeneration in higher ratios occurs in cementum and in lower ratios in alveolar bone. | Soudi A et al. 2021[7]—Mesenchymal cells have the potential to be used in tissue regeneration. | |
| Padial-Molina M et al. 2019 [41]—The effect of osteogenic differentiation in vitro is confirmed. | Roato I et al. 2021 [29]—Different anatomical origins may affect the ability to differentiate into specific tissues. | |
| Melms H et al. 2020 [39]—Same osteogenic differentiation but low adipogenic differentiation periodontal ligament cells compared to pulpal ones. | Wang LH et al. 2022 [12]—Different and numerous progenitors of mesenchymal cells have been hypothesized that, depending on their potential, they can be used for wound healing and even for partial recovery of organ function. | |
| Ma L et al. 2019 [19]—Cells of pulp origin are more resistant to lipopolysaccharide-induced apoptosis compared to those of bone marrow origin and are therefore used for periodontal regeneration more. | 0 article | |
| Lee E et al. 2021 [40]—Lack of hemopoietic markers in pulp cells, cementum-attached protein is not found in any of the types of oral mesenchymal cells. | 0 article | |
| Total | 6 articles—16% | 4 articles—11% |
In Table 6, only the articles with information about mesenchymal cells of both pulpal and periodontal origin are analyzed. In this table, again depending on the purpose of the study, concrete or effective results are shown based on the purpose of the study of the article with an orientation about adding cellular effect to mesenchymal cells of periodontal origin.
Articles on adding cellular effects of mesenchymal cells originating from periodontal tissues
| Purpose of the study | Concrete results | Evasive results |
|---|---|---|
| Adding cellular effect | Cao HL et al. 2020 [28]—Allogeneic fibrin clot contains growth factors and cytokines sufficient to induce osteogenic/cementogenic differentiation of pulp cells and periodontal ligaments in vitro. | Ahuja A et al. 2022 [24]—The usability of bioactive substances needs more studies in this field. |
| Sharifi S et al. 2020 [31]—Nano-hydroxyapatite gelatin fibers can increase the proliferative effect of cells. | 0 article | |
| Kim YH et al. 2020 [38]—Lidocaine and bupivacaine negatively affect differentiation ability; bupivacaine is less effective than lidocaine. | 0 article | |
| Total | 3 articles—8% | 1 article—3% |
Table 7 shows in a quantitative way the results collected based on the classification of articles according to the purpose of the study presented in these articles and concrete or evasive results on the application of mesenchymal cells of periodontal origin.
The data summarized from the Tables 3–6 above on mesenchymal cells of periodontal origin
| Purpose of study | Concrete results | Evasive results |
|---|---|---|
| Role of cells | 4 articles [11, 14, 25, 26]—11% | 4 articles [2, 6, 8, 10]—11% |
| Mechanism of action | 4 articles [3–5, 43]—11% | 0 article—0% |
| Field of application | 6 articles [16, 19, 20, 39–41]—16% | 4 articles [7, 12, 15, 29]—11% |
| Adding cellular effect | 3 articles [28, 31, 38]—8% | 1 article [24]—3% |
| Total | 17 articles—45% | 9 articles—24% |
It should be noted that the percentages of the total number of articles are based on the total number of articles and not by adding the percentages according to the relevant columns of the table
Discussion
Based on the data in Table 1, it is observed that the tendency to analyze the positive or negative effects of mesenchymal cells originating from the pulp of the tooth is against the application of various substances or medications with the aim to increase adhesion, migration, and differentiation properties of mesenchymal cells [9, 21]. At first sight, only anti-inflammatory and anti-pain medications, which are indicated after almost any oral bone surgical procedure, were analyzed. Positive effects of aspirin are mentioned in 2018 [22] and 2021 [36], in articles that emphasize that these effects should be further analyzed. Non-steroidal anti-inflammatory drugs with positive effects are mentioned, but diclofenac is excluded as it has a negative effect. Paracetamol was also mentioned as having positive effects on mesenchymal cells [9].
The potential therapeutic significance of mesenchymal cells originating from pulp may be disclosed if the evasive and concrete data gathered from the chosen papers are integrated, as indicated by Table 1. When combined with aspirin [36] which enhances the differentiation effect and increases the ability of osteointegration [13] mesenchymal cells of pulp origin can be used to treat bone defects resulting from periodontitis pathology [34] even when the pathology is still active [37]. When compared to applying artificial bone or autograft bone to fill bone defects produced by trauma, this approach is far less expensive, and the savings increase when mesenchymal cells from one person to another are employed as the graft material. Particularly for mesenchymal cells derived from pulp, this is one of the crucial clinical components of the application. On the other hand, mesenchymal cells [13], which differ from one another in that they have osteogenic effects in addition to their anti-inflammatory properties, continue to have this property even when these cells are obtained from inflamed pulps [23]. This property can be enhanced by local application of ibuprofen or paracetamol [13]. It is recognized that the number of partially inflamed pulps that an endodontic specialist can extract each day can be utilized to determine how much periodontal regeneration will cost. Since an endodontic specialist can extract a certain number of partially inflamed pulps each day, it is known that the only way to keep periodontal regeneration costs down is to coordinate dental procedures between a specialist in periodontology and a specialist in endodontics.
As for collagen-hydroxyapatite networks or membranes [21, 44], there are data that show an effect as long as the application of mesenchymal cells will be performed in periodontal lesions, so the field of their application is defined. Again, from the data in Table 2, it is shown that the mesenchymal cells originating from the pulp of the tooth have differentiating effects, both odontogenic [17] and osteogenic [13, 23, 34, 37]. Based on these data, it is understood that the main weight of application of the cells mesenchymal of pulpal origin would have it in the accompanying bone lesions of periodontal diseases, more than in the regeneration of the pulp itself in necrotic teeth.
There is a lack of an orientation of published studies against a co-operation of endodontics and periodontology as two specialties that can benefit from protocols designed for the simultaneous treatment of two dental pathologies with different characters of these specialties. It is precisely in the data of Table 2 that a disconnection of these specialties is clearly visible, but it is precisely this table that gives in concrete values the most expressed orientation of studies with concrete results for the clinical application of mesenchymal cells of pulpal origin.
Based on the data in Table 5, it is noted that the mesenchymal cells of periodontal origin in various research studies are intended to be analyzed for the field of application; there are 3 studies in a total of 4 that belong to this field [3, 15, 20, 40]. But among these studies, it draws attention to the fact that mesenchymal cells originating from periapical lesions can also be used for pulp regeneration [20].
From the data of the articles presented in Table 3, a comparative picture is also given between mesenchymal cells of pulpal origin and mesenchymal cells of periodontal origin. In this table, from the included articles, it is emphasized that the osteogenic ability of mesenchymal cells of periodontal origin is higher than that of mesenchymal cells of pulpal origin [26]; but be careful that these cells must not originate from an inflamed periodontal area [11], as happened in cases of application of mesenchymal cells originating from inflamed pulps [23]. So, if they are applied by the same specialist of periodontology for the same periodontal tissue regeneration procedure or for periodontal bone defects, two active periodontal surgical areas must be opened: one tooth with inflamed periodontium and the other tooth with healthy periodontium. Here comes the key to solving the situation when the data from an article says that cells can be taken even from the gingiva as they show higher clonogenicity [14].
Based on data from Tables 3–6, it seems that the role of mesenchymal cells originating from the pulp of the tooth and the periodontal ligament is seen as a comparison between these cells, emphasizing that the osteogenic and adipogenic differentiation of cells from the periodontal ligament [10, 14, 25] is higher in the cells from the pulp, but be careful; these abilities are affected if the area from which they are taken is inflamed [2, 11], but they are not affected if the area where they are placed is inflamed [6]. Mesenchymal cells act actively with exosomes [4, 5] or with markers of cell proliferation [43] to express the osteogenic, anti-inflammatory, and angiogenic effects based on inter-cellular communication. The different origins of cells may affect the ability to differentiate into specific tissues [29] but the ability to differentiate from periodontal ligament cells to pulp cells has already been confirmed [16, 19, 39, 41]. The main purpose of using mesenchymal cells is to partially regain the function of an organ [12]. The addition of growth factors, cytokines, or active substances induces osteogenic differentiation of mesenchymal cells [24, 28, 31], but beware of the prior use of dental local anesthetics [38].
Both endodontic and periodontal pathology, such as apical periodontitis, but also other periapical processes can be “cured” by the application of periodontal mesenchymal cells [43]. This element can also be related to the fact that despite perfect endodontic fillings, there are periapical processes that persist over time as a result of the precipitation of cholesterol crystals in the bone resorption lacunae as a result of local endodontic inflammation. The application of mesenchymal cells of periodontal origin would be a good clinical solution for these processes [43].
Analyzing the data on the role of mesenchymal cells of pulpal or periodontal origin, it is noticed that most of the articles are oriented around the application of cells of periodontal origin. In the selected articles, there are almost 8 articles [2, 6, 8, 10, 11, 14, 25, 26] against 0 article for mesenchymal cells of pulpal origin. This can be explained by the technique of obtaining mesenchymal cells since access to periodontal tissues is easier, but the cost of obtaining mesenchymal cells in pulpal tissues is more reduced. This element can serve as an indication for further developments in this field.
The mechanism of action of mesenchymal cells of pulpal origin is oriented around the receptors that these cells contain [3], while for mesenchymal cells of periodontal origin, this type of mechanism is twofold between the receptors and the proteins that mesenchymal cells produce to promote the regeneration of their tissue [4, 5, 43].
It is noticeable that when we talk about increasing the cellular effect, then it is the mesenchymal cells of pulpal origin, about which there are many articles where concrete positive effects are discussed [9, 21, 22, 30, 44], while if we talk about the field of applications of mesenchymal cells of oral origin, obviously mesenchymal cells of periodontal origin are the ones that are applied the most [16, 19, 20, 39–41].
The addition of growth factors or cytokines [28] or the addition of nano-hydroxyapatite gelatin fibers increase the proliferative regenerative capacity of cells of periodontal origin, but caution is required with the application of local anesthetics, as they significantly reduce this capacity, emphasizing that bupivacaine is more indicated than lidocaine [38]. This is another clinical result extracted from the articles selected for this review study, presented in the data in Table 6.
Mesenchymal cells of pulpal origin can be obtained without loss to the patient and without fatigue to the dental professional during a routine procedure of pulp extirpation, compared to periodontal cells that are found on the root surface of a tooth. Simply from the positioning of these cells, the role of the partially living pulp tissue excised from the tooth with endodontic pathology is understood.
But thinking once again of the high number of inflamed or partially inflamed pulps that an endodontist can remove in days, weeks, or months and the potential for differentiation and proliferation of mesenchymal cells originating from these pulps, at least for the application of dental procedures, perhaps limited only to periodontal ones, the role of mesenchymal cells of pulpal origin in the “otherization” and adaptation of periodontal surgical treatment protocols is somewhat understandable. “Alternation” could bring another clinical approach, especially in the marketing of artificial bone grafts.
Thinking about how many interventions a periodontologist can do per week or month with the sole purpose of filling bone defects or periodontal tissue regeneration, imagining the operating field where, in addition to a tooth affected by periodontal disease, there is also a tooth with a healthy periodontium, the role of mesenchymal cells of periodontal origin, which have the ability to regenerate tissues [12] or organs [15], can be understood, far from the operating area of the periodontologist.
These last two points of view are relatively cost-reduced since the continuing education of field specialists, endodontists, and periodontologists on the method of approach and the protocol of obtaining mesenchymal cells of respectively pulpal or periodontal origin can increase the field of application and add new possibilities in tissue regeneration technology [7, 12, 15, 24].
Minimal intervention with minimal effort added by the specialist, with the right professional training, and above all, with the good desire to do something different and better, there can be a completely different future in this field.
Declarations
Acknowledgments
Acknowledgments belong to our family. Henri and Hera are our motivation in the field of scientific research.
Author contributions
IR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Writing—original draft, Writing—review & editing. MK: Methodology, Resources. MD: Conceptualization, Project administration. VO: Formal analysis, Investigation, Resources, Supervision, Validation.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author.
Funding
Not applicable.
Copyright
© The Author(s) 2024.