Affiliation:
1Oral Medicine Department, Faculty of Dentistry, Damascus University, Damascus PO Box 30621, Syria
Email: ayadagha96@gmail.com
ORCID: https://orcid.org/0009-0008-4104-6829
Affiliation:
1Oral Medicine Department, Faculty of Dentistry, Damascus University, Damascus PO Box 30621, Syria
Affiliation:
2Periodontology Department, Faculty of Dentistry, Damascus University, Damascus PO Box 30621, Syria
Affiliation:
3Oncology Department, Faculty of Medicine, Damascus University, Damascus PO Box 30621, Syria
Explor Med. 2024;5:843–851 DOI: https://doi.org/10.37349/emed.2024.00259
Received: September 05, 2024 Accepted: November 04, 2024 Published: November 14, 2024
Academic Editor: Giuseppe Minervini, University of Campania “Luigi Vanvitelli”, Italy
The article belongs to the special issue Oral Health Interconnections and Multidisciplinary Approaches
Combined treatment of photobiomodulation therapy and pilocarpine hydrochloride (Salagen) helped a patient suffering from severe oral mucositis and malnutrition resulting from radiochemotherapy for head and neck cancers. This treatment sped up the patient’s healing process and improved his quality of life by stimulating cells and increasing saliva secretion. The severity of oral mucositis was measured according to the World Health Organization (WHO) scale and the oral mucositis assessment scale. This case report highlighted a new combination treatment that may be the key to successfully managing severe oral mucositis during radiochemotherapy without the need to stop or modify cancer therapy.
Head and neck cancer is a general term that involves epithelial malignancies that grow in the sinuses, nasal cavity, oral cavity, lips, larynx, throat, and salivary glands [1]. Nasopharyngeal cancer is one of the head and neck cancers, where malignant cells form in the nasopharyngeal tissues, causing many symptoms such as difficulties hearing, speaking, and/or breathing [2]. There are three types of standard treatment for patients with nasopharyngeal cancer: radiation therapy, chemotherapy, and surgery, also those treatments can be combined depending on the clinical data of the patient and the stage of cancer [1].
Since the previous treatments slow or stop the growth of rapidly growing cells such as cancer cells, they also affect the growth of healthy cells that are rapidly dividing, such as oral lining cells [3]. Therefore, many adverse effects may manifest, including oral mucositis (OM), odynophagia, taste alteration, xerostomia, and nutritional deficiencies [4]. From a dental perspective, dental hypersensitivity and hyposalivation are frequently observed. These complications can result in compromised oral hygiene and reduced salivary pH, potentially leading to radiation caries in the initial stages. Moreover, they elevate the risk of acute, severe dental decay and osteonecrosis, presenting a persistent threat to patients’ long-term health [5].
OM is a clinically significant complication associated with both radiotherapy and chemotherapy in cancer treatment. The presentation of OM varies depending on the therapeutic modality. Radiotherapy-induced OM predominantly affects the soft palate, followed by the hypopharynx, floor of the mouth, buccal mucosa, tongue, and lips. Typically, it emerges during the third week of treatment, with a duration spanning 7 to 98 days. Clinically, it presents with intense pain, dysphagia, and dysarthria, often necessitating interruptions in cancer therapy. Moreover, patients experience increased bleeding during routine oral hygiene due to mucosal fragility [6, 7]; chemotherapy-induced OM manifests within 5 to 14 days post-treatment initiation. It initially presents as erythema, progressing to ulceration with the formation of a fibrinous pseudomembrane. Lesions predominantly affect non-keratinized mucosal surfaces, including the soft palate, buccal mucosa, and the lateral and ventral aspects of the tongue [7, 8]. A higher incidence of OM is observed with chemotherapeutic agents such as 5-fluorouracil and methotrexate [9].
The pathological developments of OM involve five successive mechanistically linked stages: initiation, activation, signal amplification, ulceration, and healing.
Mucositis initiation is caused by radiotherapy and/or chemotherapy, leading to tissue damage, release of reactive oxygen species, DNA damage, and cell death [10]. DNA strand damages activate the apoptotic process, regulated by p53 activation and increased caspase 3, leading to the release of endogenous damage-associated molecular patterns (DAMPs) from dead cells [11].
Activation stage: the injured mucosal cells promote the transcription of genes involved in the process, and the nuclear factor-κB (NF-κB) modulates over 200 genes associated with inflammation and stress responses [12].
The amplification stage: the injury signal is amplified through the release of TNF-α, which activates MAPK to sustain NF-κB activity, amplifying the initial damage signaling through positive-feedback loop mechanisms [10].
The ulceration phase: the integrity of the mucosa and submucosa is compromised, leading to pain and requiring caregiver management. Microorganisms invade the tissue, causing an inflammation response and tissue damage. Patients may develop bacteremia or septicemia, mainly caused by streptococci and staphylococci. Mucositis is an acute condition that resolves once anticancer treatment ends, and the healing process promotes tissue re-epithelialization [10].
Healing stage: ulcers spontaneously heal but may recur due to lingering blood vessel formation. Epithelial growth and the restoration of local microbial flora characterize this stage [13].
Various validated scales for assessing the severity of OM have been introduced in the scientific literature. The OM assessment scale (OMAS) and the World Health Organization (WHO) OM grading scale are prominent among these, both widely utilized for clinical evaluation and research purposes. Additionally, the LENT-SOMA scale has been proposed as a tool for evaluating xerostomia, offering a standardized approach to quantify radiation-induced salivary gland dysfunction. These assessment tools are critical for consistent clinical diagnosis, monitoring, and treatment optimization.
The OMAS calculates the mean mucositis score using the formula: (Σ ui/nu) + (Σ ei/ne), where “nu” and “ne” represent the number of ulceration and erythema sites, respectively, and “ui, ei” represents the sum of ulceration and erythema values, respectively. The resulting score ranges from 0 to 5, with a score of 5 indicating the most severe presentation, characterized by extensive erythema and ulceration [14]. The WHO OM grading scale grades mucositis severity on a scale of 0 to 4: a score of 0 reflects no signs of mucositis; while a score of 1 indicates mild oral soreness or erythema; a score of 2 corresponds to moderate erythema or ulceration with the ability to tolerate a solid diet; a score of 3 reflects severe ulceration with only liquids tolerated; and a score of 4 denotes a life-threatening condition where oral feeding becomes impossible [15]. Additionally, the LENT-SOMA scale, used to assess xerostomia, grades severity from 1 to 4, with 1 indicating normal salivary moisture, 2 representing scant saliva, 3 indicating the absence of moisture with sticky, viscous saliva, and 4 reflecting a complete absence of moisture with a coated mucosal surface [16].
To prevent and manage OM, various therapeutic approaches have been investigated, including cryotherapy, anti-inflammatory agents, growth factors, analgesics, and natural products [17–19]. One of the key recommendations from the Multinational Association of Supportive Care in Cancer (MASCC) for the management of OM, particularly in patients undergoing radiation therapy or those with head and neck cancers, is the use of low-level laser therapy (LLLT), now referred to as photobiomodulation therapy (PBMT) [20, 21]. PBMT is a non-invasive treatment modality where cellular cytochromes, acting as primary photoreceptors, absorb light at specific wavelengths. This absorption stimulates the mitochondrial respiratory chain, leading to increased production of adenosine triphosphate (ATP), the cell’s primary energy molecule. The activation of these pathways triggers a cascade of biochemical responses, including anti-inflammatory and anti-edematous effects, enhanced vascularization, and tissue repair. Furthermore, PBMT exerts analgesic effects by modulating pain perception, promoting the release of endorphins, and altering neural conduction pathways [22]. Consequently, PBMT has been shown to significantly alleviate the severity and symptoms of OM [21, 23].
Another treatment option is pilocarpine hydrochloride (Salagen) which is the most common drug, approved by the FDA, for treating Sjogren syndrome and alleviating the symptoms of oral dehydration caused by radiation therapy [24]. Patients treated with Salagen experienced an improvement in whole saliva, considering that the sensation of oral wetness and improvements in sore mouth and speaking are likely to be influenced by minor salivary gland secretions [25].
This case report aimed to highlight a new effective combination therapy using photobiomodulation and pilocarpine hydrochloride for the management of severe OM caused by radiochemotherapy in head and neck cancer patients, which often leads to discontinuation of cancer treatment due to the patient’s malnutrition, thus threatening the patient’s life.
A 60-year-old male patient was referred from the radiology department at Al-Biruni hospital to the Oral Medicine Department in the Faculty of Dental Medicine at Damascus University.
The patient was in a life-threatening situation, requiring the cessation of cancer therapy and immediate hospitalization. The main complaint was severe oral pain, oral dryness, burning mouth sensation, and inability to talk, sleep, eat, or drink except for a small amount of water, leading to malnutrition.
He was previously diagnosed with nasopharyngeal cancer and was undergoing radiotherapy protocol included 70 Gy distributed over 34 sessions along with once-a-week chemotherapy including docetaxel 80 (Taxotere®; Sanofi, Jeddah, ST: Tahliah, KSA), carboplatin 450 (carboplatin; Sanofi, Jeddah, ST: Tahliah, KSA), fluorouracil (5FU) 500 (5-fluorouracil; Sanofi, Jeddah, ST: Tahliah, KSA), also nystatin oral suspension and Listerine mouthwash were used as a palliative adjuvant therapy.
During the extraoral examination, the patient appeared pale; he was fatigued and had lymphedema affecting the submandibular and superficial cervical nodes.
The intraoral examination revealed more than 3 cm2 of ulcers and severe erythema covered with a layer of fibrin, on the hard and soft palate as shown in Figure 1A. This condition scored five and four on the OMAS and the WHO scales, respectively, marking the worst score on these scales. In addition to the absence of moisture in the oral mucosa, which was rated as fourth grade on the LENT-SOMA scale.
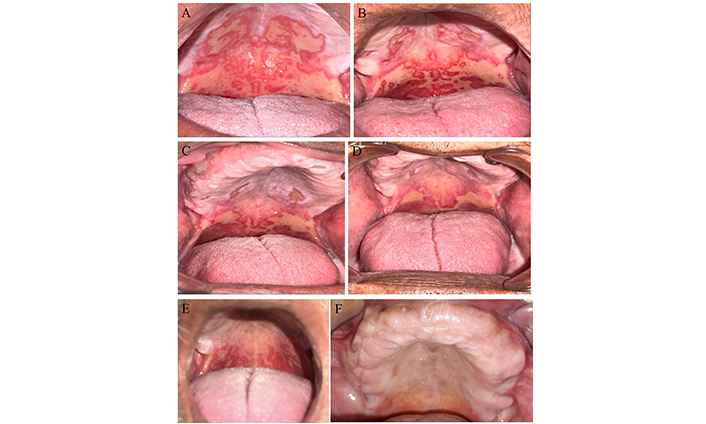
The patient’s condition at each stage. (A) Prior to the combined treatment session, the initial diagnosis revealed over 3 cm2 of ulcers and severe erythema, indicating severe oral mucositis (OMAS = 5) due to radiochemotherapy in a head and neck cancer patient; (B) second session, 72 hours after the initial combined treatment session, the ulcers still measured over 3 cm2, but the hard palate erythema was not severe (OMAS = 4.5); (C) third session, 72 hours after the second combined treatment session, the ulcers on the hard palate had improved and measured between 1–3 cm2 with no severe erythema (OMAS = 3.5); (D) fourth session, 72 hours after the third combined treatment session, the hard palate ulcers measured less than 1 cm2, and the soft palate measured more than 3 cm2 with no severe erythema (OMAS = 3); (E) the last treatment session, 72 hours after the fourth combined treatment session, the ulcers subsided, but no severe erythema remained (OMAS = 1); (F) four weeks’ follow-up, the oral mucosa revealed no ulcers or erythema (OMAS = 0). OMAS: oral mucositis assessment scale
According to the patient’s clinical, medical, and family history, he was a heavy smoker diagnosed with nasopharyngeal cancer without any other systemic diseases. He suffered from OM and xerostomia induced by 19 sessions of radiochemotherapy and still needed 15 sessions.
After the patient was referred to the oral medicine department, the patient was informed about the treatment and its side effects. The patient’s written consent was taken before starting the treatment of choice: the combined treatment of pilocarpine hydrochloride (Salagen; B27003 ADEKA, Istanbul-Maslak, ST:23, Turkey); 5 mg tablet three times a day, an hour before meals [26], and the PBMT three times a week while continuing his radio-chemotherapy sessions. Also, he was instructed to avoid sour and spicy foods and to eat rich, nutritious soups until the last session of the treatment plan.
The diode laser [arsenide gallium aluminum (AsGaAl)] device from PIOON company, model MER-G10 in the oral medicine department in the faculty of dental medicine at Damascus University, was utilized once every 72 hours according to the following protocol proposed by Zecha et al. [27].
Intraoral application on minor salivary glands with a wavelength of 650 nm, a power of 40 mW, a time of 25 s, a spot size of 0.5 cm2, an energy density per point 2 J/cm2, one joule per point, and power density of 80 mW/cm2, in continuous waves and contact mode on the following points: a point on each labial commissure, 8 points on the upper labial mucosa, 8 points on the lower labial mucosa, 12 points on each buccal mucosa, 12 points on the hard palate, 4 points on the soft palate, 6 points on each border of the tongue, 6 points on the ventral surface of the tongue, and 4 points on the mouth floor.
After completing the treatment, the patient underwent five sessions of PBMT and also took Salagen for 15 days. The levels of OM and dryness were assessed 72 hours after each diode laser session. The results were presented in Table 1, where R1 indicated the scores prior to the first treatment session Figure 1A. R2 referred to 72 hours after the first treatment session Figure 1B. The next two sessions were seventy-two hours apart, as shown in Figure 1C and D, respectively. The severity of OM significantly decreased, as evidenced by an improvement in the OMAS scale score from 5 to 1, and a reduction in the WHO scale grade from 4 to 1. The LENT-SOMA scale grade also improved from 4 to 2, The patient’s overall condition improved, particularly in terms of nutritional intake, allowing him to continue receiving radio-chemotherapy. Consequently, as shown in Figure 1E, the ulcers were completely healed, and the majority of the oral mucosa’s health was restored. The patient was scheduled for follow-up evaluations at two and four weeks post-treatment. Although the patient missed the two-week appointment, a phone consultation indicated the resolution of pain and the absence of sleep disturbances. At the four-week follow-up, clinical examination as highlighted in Figure 1F, revealed that the oral mucosa had returned to a healthy state, and the patient reported significant improvements in overall quality of life.
Results of the scales of oral mucositis and xerostomia
| Scales | R1 | R2 | R3 | R4 | R5 |
| Oral mucositis assessment scale (OMAS) | 5 | 4.5 | 3.5 | 3 | 1 |
| WHO oral mucositis grading scale | 4 | 3 | 2 | 2 | 1 |
| LENT-SOMA | 4 | 4 | 3 | 3 | 2 |
R1: the first session before starting the combined treatment; R2: 72 h after the first combined treatment session; R3: 72 h after the second combined session; R4: 72 h after the third combined session: R5: 72 h after the fourth combined session (the last session)
OM is one of the most frequent and distressing side effects experienced by head and neck cancer patients undergoing radiochemotherapy. Although mucositis usually starts asymptomatic, it can eventually cause redness, burning sensations, and hypersensitivity, especially to hot and spicy food. In more critical cases, oral ulcers may develop, causing dysphagia and reducing nutrition intake [28]. Clinically, OM progresses from erythema and ulcers to pain and bleeding, with mucosal breakdown increasing the risk of local and systemic infections, which may significantly impact the quality of life [29]. The incidence of mucositis varies by treatment modality, affecting 20–40% of patients receiving conventional chemotherapy, 60–85% of those undergoing hematopoietic stem cell transplantation (HSCT), and nearly all individuals receiving radiation therapy for head and neck cancers [30]. Consequently, OM may necessitate dosage reductions or discontinuation of radiotherapy and/or chemotherapy, ultimately diminishing the patient’s survival prospects [31].
Based on this case report, the patient experienced severe OM with ulcers over 3 cm2 and severe erythema, which scored 5 on the OMAS; the oral feeding was impossible and life-threatening which scored 4 on the WHO OM grading scale; also, the patient’s suffered from a complete absence of moisture with a coated mucosal surface which graded 4 according to the LENT-SOMA scale. Given the critical nature of the case and the necessity to continue cancer treatment without interruption, it was imperative to identify rapid and safe interventions to manage OM, thereby ensuring the patient’s ability to adequate nutritional intake. Therefore, several treatment strategies for managing OM during cancer treatment are recommended according to the National Cancer Institute (NCI), including basic oral care, oral rinses, anti-inflammatory agents, cryotherapy, LLLT, hydration, and lubrication of the oral mucosa [19]. Our treatment strategy involved a well-established regimen that has been recommended for over a decade for the prevention and management of OM, as well as for the mitigation of associated pain. However, to date, consensus on the development of standardized protocols remains limited. So, this approach was further augmented by an additional intervention aimed at stimulating salivary production and enhancing masticatory function, thereby improving the patient’s overall ability to chew and ingest food. The first treatment is PBMT, which is recommended for the management of OM due to several supporting factors. Consistent evidence from a limited number of high-quality studies indicated that red and infrared PBMT applied at doses between 1 and 6 joules per point can partially prevent the development of cancer therapy-induced OM. Moreover, PBMT has been shown to significantly alleviate pain and promote healing [32]. Additionally, animal studies have demonstrated that PBMT modulates saliva production and regulates oxidative mechanisms, thereby reducing the inflammatory response [33, 34]. Despite that, several studies have suggested that PBMT is not effective in preventing OM or enhancing the quality of life in cancer patients undergoing radiochemotherapy for head and neck cancer. This is mainly due to variations in laser protocols [20, 35, 36].
The second treatment strategy is the hydration of the oral mucosa. Since the saliva contains several proteins such as fibroblast growth factor (FGF) and epidermal growth factor (EGF) that promote tissue repair and wound healing, it has been suggested that decreased saliva flow may cause toxic damage to oral mucosal cells and delay their healing [37]. Therefore, pilocarpine hydrochloride (Salagen tablet) was used in this case, since it is the only drug product that has gained approval in both the USA and Europe and it is used to manage xerostomia caused by radiation [24]; it works as a cholinergic parasympathetic agent that binds to muscarinic acetylcholine receptor 3 (M3R) located in the smooth muscle cells, acinus cells of the salivary glands, and gastric glands, causing direct stimulation for these cells. Thus, it reduces the symptoms of mouth dryness [38, 39]. Also, studies have shown that pilocarpine helps decrease the incidence of third-grade OM and improves patients’ quality of life [40, 41]. In conclusion, this case study demonstrated the successful outcome of a novel combined treatment using PBMT and pilocarpine hydrochloride for managing severe OM. This treatment improved nutritional intake and the quality of life, without requiring the interruption of radiochemotherapy in patients with head and neck cancer.
LLLT: low-level laser therapy
NF-κB: nuclear factor-κB
OM: oral mucositis
OMAS: oral mucositis assessment scale
PBMT: photobiomodulation therapy
WHO: World Health Organization
ADA: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Funding acquisition, Methodology. MAH: Conceptualization, Investigation, Writing—review & editing, Resources. AA: Validation, Visualization. MB: Validation, Supervision.
The authors declare that they have no conflicts of interest.
This study conforms to the Declaration of Helsinki ethical principles for medical research and was approved by the ethics committee at Damascus University, number 3779.
Informed consent to participate in the study was obtained from the participant.
Informed consent to publication was obtained from participant.
All datasets analyzed for this study are included in the manuscript.
The study was funded by Damascus University, and the number is DU2022-3796. The funder had no role in study design, data collection, and analysis, the decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Martina Costanzo ... Ilenia Campione
Zeina Darwich ... Chadi Azmeh
Francesca Gorassini ... Gabriele Cervino
Olha Denefil ... Natalia Tverdokhlib
Gerardo Pellegrino ... Giuseppe Lizio
Alberto Enrique Varela ... José E. Rodríguez
Aiswarya Polumatla ... Tejaswin Polepalle
Alessia Pardo ... Massimo Albanese
