Affiliation:
1School of Public Health, University of West Attica, 11521 Athens, Greece
Email: vraikou@med.uoa.gr
ORCID: https://orcid.org/0000-0002-4290-8426
Explor Med. 2024;5:870–879 DOI: https://doi.org/10.37349/emed.2024.00261
Received: July 31, 2024 Accepted: October 01, 2024 Published: November 19, 2024
Academic Editor: Sverre E. Kjeldsen, University of Oslo, Norway
The article belongs to the special issue Drug Adherence in Hypertension
Hypertension (HTN) is connected to many complications such as stroke, heart attack, heart failure, and kidney damage. Aging, lifestyle modifications, and obesity are risk factors associated with arterial HTN. On the other hand, chronic kidney disease (CKD) is a gradually progressive disease that is associated with cardiovascular disease (CVD), HTN, anemia, electrolyte imbalance, acid-base abnormalities, and bone disease. Blood pressure (BP) in hemodialysis patients shows a dynamic nature during dialysis procedures, including intradialytic hypotension and/or intradialytic HTN. Even though hypotensive events are common in hemodialysis sessions, intradialytic HTN, in which the BP increases during and/or immediately after hemodialysis, was associated with a higher mortality risk. The prevalence of intradialytic HTN has been described in 5–20% of hemodialysis treatments. The coexisting comorbidities in CKD patients need adequate pharmacological treatment. As a result, CKD patients are at a high risk for polypharmacy, which causes an elevated risk for adverse drug effects and influences non-adherence to medication. In addition, it is required individualization of medication doses adapted to the decreased renal function according to the progression of CKD. The improvement of health literacy through suitable interventions can facilitate the perception of illness, resulting in high therapy adherence in such a group of patients. This review considers the aspects of HTN management and adherence to the treatment in patients in permanent hemodialysis therapy, contributing to the determination of more effective strategies for improved treatment compliance, aiming at the prevention of CVD in this patient population.
Arterial hypertension (HTN) is a significant risk factor for heart disease and stroke, the main leading causes of death globally. It affects more than 1.2 billion people aged between 30 and 79 years with an increased prevalence rate particularly in low and middle-income countries [1, 2]. Moreover, up to 75% of patients with HTN are proved unable to control their blood pressure (BP) resulting in a high undertreated HTN, especially among older adults [3, 4]. Arterial stiffening due to aging, as an important clinical demonstration of HTN in this population, is a main pathophysiological mechanism connected to heart failure, stroke, and death [5, 6]. Arterial stiffness is a common characteristic among the patients with chronic kidney disease (CKD) [7].
The aging population in combination with a growing prevalence of diabetes, HTN, and obesity increases the impact of CKD on healthcare systems [8]. CKD is an important risk factor for cardiovascular disease (CVD) and this is associated with a higher risk of premature mortality, increased healthcare use, and a lower quality of life [9].
Antihypertensive therapy shows an improvement in BP control. However, it has been reported that the adherence rate to antihypertensive treatment ranges from 20% to 50% [10–13]. The reasons for such a low rate include old age, which is connected to decreased cognitive function or progressive depression, in addition to other factors [14].
The patients on hemodialysis treatment have the most elevated medication burden to other disease groups. Because they must attend multiple treatments to slow disease progression, manage coexisting conditions, and address complications. It has been previously reported that the non-adherence rate varies from 3 to 80% in CKD patients [15]. Non-adherence in this population of patients is related to increased mortality and an elevated healthcare cost. A previous systematic review, which considered studies applied in Medline, Embase, SCOPUS, and CINAHL regarding pre-dialysis CKD patients, noted that the most studied medication class was antihypertensives (55.6%) [16]. HTN particularly in hemodialysis patients is multifactorial and it mainly depends on hypervolemia. Moreover, BP in dialysis patients is not a stable condition, but it presents a dynamic nature during dialysis session, including intradialytic hypotension and/or intradialytic HTN. These two special changes of BP during the hemodialysis procedure were significantly associated with an increased risk of mortality in these patients [17].
Therapy adherence is defined as an active engagement of patients in a process of acceptable health-related behaviors resulting in positive treatment outcomes [18]. Therapy adherence has a potentially positive effect on renal disease, complications of hemodialysis, patients’ quality of life, and hemodialysis outcomes [19]. It includes compliance with suitable diet, medications, fluid restriction, and strict attendance at hemodialysis sessions [20]. There are different reasons for non-adherence in such patients, including sociodemographic factors, healthcare-based issues, economic barriers, polypharmacy, the big burden of disease which the patients have to endure, anxiety, and psychiatric disease which is commonly combined with renal disease, perception of the illness and perceived necessity for medication [16, 21, 22].
In this review, we address the aspects of HTN management and adherence to the treatment in patients on hemodialysis, contributing to the determination of more effective strategies for improved compliance with treatment and aiming at CVD protection in this patient population.
The increasing aging in combination with the multimorbidity of population are the main reasons why CKD shows an increased prevalence rate. Non-dialysis elderly patients have become prevalent in nephrology clinics. Despite the main probability of dying from end-stage renal disease (ESRD) being low compared to other causes of death, the risk of elderly people ending up in dialysis treatment has almost doubled in the last 25 years. The most studies used the calculation of glomerular filtration rate (GFR) to identify CKD (GFR < 60 mL/min/1.73m2). However, the additional classification according to albuminuria categories is required for the diagnosis of CKD [23].
HTN and diabetes mellitus are traditional risk factors for the development of CKD and both of them are considered potential causes for a such disease [24]. On the other hand, the increased renal tubular sodium reabsorption and activation of the renin angiotensin-aldosterone system (RAAS) may be the reasons for the initiation of HTN caused by renal function, especially when obesity is coexisted [25]. Therefore, the progression of renal disease and BP contribute both to the demonstration of HTN in CKD patients. In ESRD, HTN is a usual characteristic, and more than 85% of new patients suffer from HTN [26]. Many factors affect the demonstration of HTN in these patients including persistent hypervolemia and elevated peripheral vascular resistance. In the elderly particularly patients, in whom 3 hemodialysis sessions per week have been prescribed, BP increases according to weight gain, which they received during the interdialytic interval. Consequently, the control of extracellular volume (ECV) must be the main goal of hemodialysis treatment, because inadequate sodium and fluid removal have as an effect fluid overload, increased BP, and increased mortality. The increased plasma concentrations of angiotensin II and norepinephrine may cause elevated peripheral resistance and promote an inappropriate activation of the sympathetic nervous system [26]. HTN is directly connected with disorders in cardiac structure. Disorders of cardiac function including left ventricular hypertrophy, diastolic dysfunction, and arterial stiffness result in final adverse outcomes [27].
Increased systolic blood pressure (SBP) rather than diastolic blood pressure (DBP) and, consequently, increased pulse pressure (PP) are the main characteristics of HTN in CKD patients. Increased arterial stiffness, as it is assessed by an increased pulse wave velocity (PWV), increases SBP by wave reflection and decreases DBP, resulting in an increased PP. It has been previously reported that arterial stiffness is importantly influenced by volume overload in dialysis patients [28]. However, previously, we suggested that hydration status alterations and hypervolemia may significantly cause an increased PP in dialysis patients than the elevated PP to be a consequence of increased arterial stiffness, despite fluid overload can be associated with high arterial stiffness [29].
Nevertheless, BP shows a dynamic rather than stable nature during dialysis procedures. During dialysis treatment, BP usually is decreased, which is a common phenomenon. However, BP may be increased during and/or immediately after hemodialysis session in some patients, which is called intradialytic HTN. Intradialytic hypotension and/or intradialytic HTN are two special conditions, which were significantly associated with an elevated risk of mortality of these patients. According to previous studies, intradialytic HTN was a more important risk factor for mortality than intradialytic hypotension, which is more usually observed during dialysis procedure [17]. The prevalence of intradialytic HTN has been shown in 5–20% of hemodialysis treatments in agreement with our previous study which found the prevalence rate of intradialytic HTN to be equal to 19.7% [7].
Hemodynamic imbalance or/and a paradoxical reaction to the dialysis procedure in a subset of hemodialysis patients may explain the alterations of BP during hemodialysis treatment including either intradialytic hypotension or intradialytic HTN (Table 1) [30]. Older age, lower body weight, lower serum creatinine and albumin, and utilization of more antihypertensive medications are included in the factors that are associated with intradialytic BP rise. Small rather than big reductions of osmolarity during dialysis caused by lower albumin and pre-dialysis urea nitrogen levels may prevent the BP to be reduced. Intradialytic HTN may be additionally connected with history of HTN commonly observed in hemodialysis patients [31]. Another contributor to intradialytic BP rise may be the removal of received antihypertensive medications through used filter membrane during the hemodialysis procedure. However, it has been demonstrated that the intradialytic BP alterations were not significantly associated with the antihypertensive number, class, and dialyzability status [32]. A combination of positive sodium balance, liquids overload, activation of RAAS and sympathetic nervous system, endothelial cell dysfunction, erythropoiesis stimulating agents, and bone disease disorders was suggested to be the cause for intradialytic BP variability. Moreover, we previously studied 76 dialyzed patients with mean age of 62.2 ± 15 years and we showed that the loss of residual renal function (RRF) influenced by volume overload was associated with cardiovascular outcomes in these patients (Figure 1). The presence of a progressive inflammation/oxidative stress condition proved by increased levels of monocyte chemoattractant protein-1 (MCP-1), a chemokine, which may reflect an inflammatory environment may be an explanation for a such finding (Figure 2) [33]. We also previously showed that metabolic abnormalities, such as malnutrition/inflammation and uncontrolled metabolic acidosis status were significantly associated with the manifestation of intradialytic HTN in permanent hemodialysis treatment patients. Accelerated metabolic acidosis may reflect sodium imbalance and hemodynamic instability of these patients, as a result of volume overload, despite it is not clinically apparent (Figure 3) [7]. Furthermore, according to the findings of our same previous study, the older age, the bigger dialysis vintage, and the lower urine output may contribute to a higher and uncontrolled metabolic acidosis state in intradialytic HTN patients [7].
Reasons for intradialytic hypertension phenomenon in hemodialysis patients
| List of reasons |
|---|
| paradoxical response to the dialysis procedure |
| hemodynamic imbalance |
| older age |
| lower body weight |
| lower serum creatinine and albumin |
| utilization of more antihypertensive medications |
| history of hypertension |
| the removal of antihypertensive medications during hemodialysis treatment |
| positive sodium balance |
| volume overload |
| activation of the renin-angiotensin aldosterone and sympathetic nervous system |
| endothelial cell dysfunction |
| erythropoiesis stimulating agents |
| bone disease disorders |
| the loss of residual renal function |
| inflammation/oxidative stress condition |
| uncontrolled metabolic acidosis |
| big dialysis vintage |
| Non-adherence to some dietary and fluids restrictions |
| medications non-adherence |
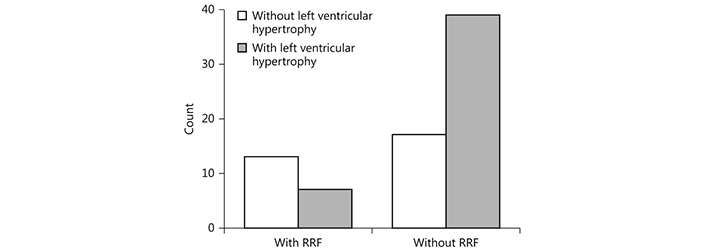
Relationship between loss of residual renal function (RRF) and left ventricular hypertrophy. χ2 = 7.4, p = 0.007. Reprinted with permission from [33], © Copyright 2017, Silverchair Publisher
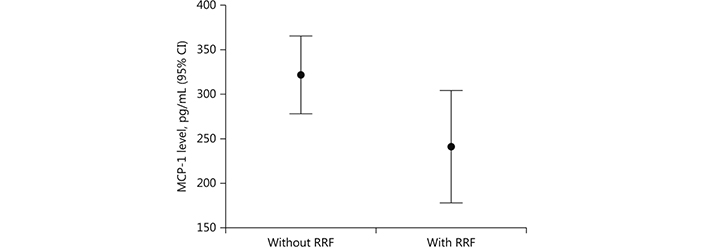
Monocyte chemoattractant protein-1 (MCP-1) levels in patients without and with residual renal function (RRF). CI: confidence interval; p = 0.04. Reprinted with permission from [33], © Copyright 2017, Silverchair Publisher
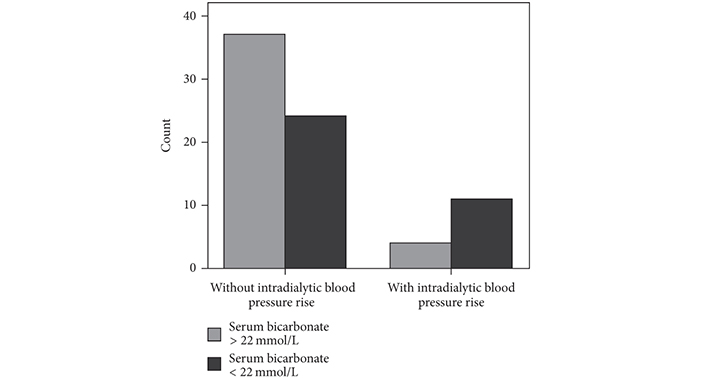
Intradialytic hypertension and metabolic acidosis. Bar chart for the association between intradialytic hypertension and metabolic acidosis state defined by serum bicarbonate less than 22 mmol/L. χ2 = 5.6, p = 0.01. Reprinted from [7] (CC BY 4.0), © Copyright 2018, Vaia D. Raikou and Despina Kyriaki
It has been already reported a significant association between uremic acidosis, HTN, and cardiovascular instability in hypertensive patients. Metabolic acidosis is combined with increased ionized plasma calcium leading to high BP, due to ionized plasma calcium having a crucial role in the regulation of BP. The high arterial stiffness in these patients may be caused by increased BP which is induced partly by uremic acidosis. In addition, a recent study in renal failure patients has shown that age, diabetes mellitus, and increased SBP are more related to vascular stiffness and vascular calcification rather than the uremic toxicity [34].
Medication adherence is defined by the World Health Organization (WHO) as the extent to which an individual acts according to recommendations provided by a healthcare professional [35]. Moreover, WHO defined five dimensions of medication adherence including the condition, the patient, the therapy, the health-system, and socio-economic domains [16]. A systematic review suggested advanced CKD was mainly associated with poorer medication adherence among condition-related factors, misconception about medications between patient-related factors, polypharmacy for therapy-related factors, the loss of confidence in the physician including in health-system-based factors, and socioeconomic factors including poor social support and lower education levels connected to poor medication adherence [16].
Elderly adults with CKD often have many comorbidities and it is required to take multiple medications including antihypertensives. It has been already reported that the medication burden in this group of patients ranges from 12–19 prescribed medications per day. These patients need to take additional medications in order to limit the progression of CKD and manage complications related to renal disease, such as anemia, metabolic disorders, hyperlipidemia, mineral and bone disorders. These abnormalities are established during the progression of kidney function [36]. In a low kidney function, glomerular filtration, tubular secretion, and reabsorption are reduced. An impaired kidney function causes many changes to the pharmacokinetic and pharmacodynamic properties of medications regarding absorption, distribution, metabolism, and their excretion, because the most drugs are mainly eliminated through the kidneys. Therefore, the likelihood of potentially life-threatening toxicities, drug-drug interactions, and adverse drug reactions is increased in this group of patients, if the dosing of medication is not adapted to current kidney function [37].
Adherence to treatment is one of the factors with a significantly positive impact on CKD and hemodialysis complications, quality of life, and hemodialysis outcomes [19]. Hemodialysis is not a perfect substitute for kidneys, despite the advanced technology and it has limited efficiency if therapy adherence is absent [38]. High adherence to pharmacological and non-pharmacological therapies such as nutrition therapy, anxiety and stress management, sleep improvement modalities, and physical exercise can improve fatigue, a common characteristic in these patients [39]. Furthermore, dialysis patients must restrict phosphorus intake, which is mainly in rich protein food, despite it is required for these patients to receive adequate protein to prevent malnutrition [40]. The additional adherence to salt restriction can reduce their thirst distress, another usual characteristic in this group of patients. Moreover, adherence to treatments for anemia can increase hemoglobin levels and oxygen transport and thereby, reduce fatigue and improve the quality of life in dialysis patients [41]. Previous study showed that the compliance with some dietary restrictions was reduced at a rate equal to 86% of patients receiving hemodialysis [42]. Another study revealed that the adherence to fluid restriction was poor at a rate of 33% of these patients [43]. It has been also shown that one-fourth of patients with ESRD had avoided hemodialysis sessions during the past month [44]. It is worthy to note that, the absence of hemodialysis sessions increases waste products in the body and increases the mortality rate [45]. Non-adherence to some dietary and fluid restrictions in combination with medications non-adherence contribute to a poor control of HTN and induce the intradialytic HTN phenomenon, due to diet and fluid overload influencing the extent of hypervolemia and metabolic acidosis status in hemodialysis patients.
A poor treatment adherence may be attributed to the polypharmacy and unpleasant taste of some medications, the complexity of treatments, and the side effects of medications [46, 47]. Recently, it has been shown that the reduction of patients’ concerns about their medications may increase the adherence. Moreover, the improvement of health literacy by suitable interventions can facilitate the perception of illness resulting in a high therapy adherence in a such group of patients [48, 49].
This review noted mechanisms regarding HTN management in hemodialysis patients and the involved complications including intradialytic HTN phenomenon. We also discussed aspects of treatment adherence related to HTN management aiming to have a reduced morbidity and mortality of these patients, as a final effect.
BP: blood pressure
CKD: chronic kidney disease
CVD: cardiovascular disease
ESRD: end-stage renal disease
HTN: hypertension
PP: pulse pressure
SBP: systolic blood pressure
VR: Writing—original draft, Data curation. SG: Conceptualization, Writing—review & editing, Visualization.
The authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3842
Download: 49
Times Cited: 0
Sameen Abbas ... Sohail Kamran
Claudio Tomasino, Marco Tomasino
Eirik Olsen ... Camilla L. Søraas
Shawna D. Nesbitt
Aaron Walsh ... Ranjit Philip
