Abstract
Aim:
This study aimed to explore the feasibility and preliminary efficacy of cinobufacini in patients with oral premalignant lesions (OPLs).
Methods:
Patients with histologically confirmed OPLs participated in an open-label uncontrolled pilot clinical study and received a 4-week or 12-week treatment, the efficacy and safety of cinobufacini for the treatment of OPLs were assessed.
Results:
During the treatment course ranging from 4 weeks to 12 weeks, no one withdrew because of adverse effects, no one had clinical or histologic progressive disease. Of the 8 participants who took cinobufacini for 12 weeks, one had a complete clinical response, and 4 had a complete histologic response. Of note, 9 participants had varying degrees of pain reduction.
Conclusions:
This small pilot study supports the feasibility of a larger clinical trial to evaluate the efficacy of cinobufacini in the treatment of OPLs [Chinese Clinical Trial Registry (chictr.org.cn) identifier: ChiCTR2300068529].
Keywords
Cinobufacini, oral potentially malignant disorders, potentially premalignant oral epithelial lesions, oral lichen planus, oral leukoplakiaIntroduction
Oral cancer represents a significant global health concern, with over 260,000 new cases reported annually worldwide, a considerable proportion of the cases emerge from oral premalignant lesions (OPLs) [1], so effective control or treatment of OPLs is important to prevent the occurrence of oral cancer [2]. By now, various systemic agents (e.g., vitamin A, beta-carotene, and metformin) have been reported in OPLs chemoprevention studies, however, their efficacy is not widely recognized due to evident toxicity, unfavorable clinical response, or high recurrence rates [3–6].
Cinobufacini is a traditional Chinese medicine extracted from Venenum Bufonis [7], it exhibits antitumor activities in many human cancers, including oral cancer [8–11]. In 2018, cinobufacini was listed in the National Essential Drugs of China [12]. The anticancer mechanisms related to cinobufacini include activation of extrinsic and intrinsic pathways of apoptosis, inhibition of oncogenic signaling, and angiogenesis [13]. Importantly, no significant toxicity was observed in clinical use of cinobufacini even at doses up to eight times the typical dose [14], making it more acceptable to patients than other chemopreventive agents for cancer treatment. Due to its anticancer activity and safety, we conducted a pilot study to assess the feasibility and preliminary efficacy of cinobufacini in patients with OPLs.
Materials and methods
The drug product was commercially available cinobufacini tablets manufactured by Anhui Huarun Jinchan Pharmaceutical Co., Ltd (batch number: 210317, 211117). Each tablet contained 0.3 g cinobufacini extract. Fifteen patients comprising 9 females and 6 males were recruited to the pilot study from Stomatological Hospital, the Fourth Military Medical University. The selection of biopsy site was referred to the method reported by Gutkind et al. [5], and pathological change was evaluated by hematoxylin-eosin (HE) staining. The inclusion criterion was the presence of histologically confirmed OPLs with mild, moderate, or severe dysplasia. All patients had no uncontrolled systemic diseases, and did not use immune agents in the past three months. We also excluded cases where the lesion areas were smaller than 1 cm × 1 cm. During the treatment, no other medications, including topical medicine were administered. Continuous variables were analysed by Student’s t-test, P < 0.05 was considered as statistically significant. The Trial Registration Number: ChiCTR2300068529.
Results
Fifteen patients were recruited to the pilot study, the detailed patient demographics are shown in Table 1. Pretreatment laboratory evaluation parameters included complete blood count, platelet count, blood glucose, blood lipid levels, liver function, and renal function. Participants were administered cinobufacini orally at a dosage of 0.6 g thrice daily for 4 weeks. Safety assessment was performed at 24 h, one week, two weeks post-treatment, and adverse events occurring during the course of the clinical trial were recorded. Participants underwent hematological and blood biochemical assessments 4 weeks after the procedure to further evaluate the safety of this trial.
Patient profiles
| ID | Sex | Age | Duration(years) | Smoking history | Alcohol consumption | Location and disorders | Baseline histology | Treatment duration (weeks) |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 57 | 10 | Former smokers | Some days | Tongue, OLP | Moderate | 4 |
| 2 | Male | 33 | 2 | Former smokers | Some days | Tongue, OL/OE | Mild | 4 |
| 3 | Female | 54 | 4 | No | Not at all | Gingiva, OL | Mild | 4 |
| 4 | Male | 57 | 3 | Former smokers | Some days | Gingiva, OL | Mild | 4 |
| 5 | Female | 60 | 1 | No | Not at all | Gingiva, OL | Mild | 4 |
| 6 | Female | 51 | 2 | No | Not at all | Tongue, OL/OE | Severe | 4 |
| 7 | Female | 49 | 3 | No | Not at all | Tongue, OL/OE | Moderate | 4 |
| 8 | Female | 52 | 1 | No | Not at all | Tongue, OE | Moderate | 12 |
| 9 | Female | 65 | 2 | No | Not at all | Tongue, OL | Mild | 12 |
| 10 | Male | 55 | 10 | Former smokers | Some days | Buccal, OL | Moderate | 12 |
| 11 | Male | 44 | 7 | No | Not at all | Tongue, OL/OE | Mild | 12 |
| 12 | Female | 64 | 5 | No | Not at all | Buccal, OLP | Mild | 12 |
| 13 | Male | 45 | 2 | No | Some days | Tongue, OL | Mild | 12 |
| 14 | Female | 59 | 1 | No | Not at all | Buccal, OL | Severe | 12 |
| 15 | Female | 74 | 6 | No | Not at all | Buccal, OL | Moderate | 12 |
OLP: oral lichen planus; OL: oral leukoplakia; OE: erythroplakia
Clinical response was categorized as follows: complete response, total disappearance of all lesions; partial response, reduction of lesion size by at least 50% in diameter; stable disease, reduction of lesion size by less than 50% in diameter or no change; progressive disease, greater lesion size or appearance of new lesions. Numerical rating scale (NRS) was used as an indicator to assess the intensity of pain. Participants were requested to select a number ranging from 0 to 10 to indicate their pain level over the preceding four weeks, whereby 0 indicated no pain, 1–3 signified mild pain, 4–6 denoted moderate pain, 7–9 indicated more severe pain, and the maximum score of 10 represented severe unbearable pain. After the completion of the 4-week trial, participants were given the option to independently choose whether to continue the treatment. The participants who continued treatment for 12 weeks would undergo a pathological evaluation. Histologic evaluation of all biopsies was performed independently by two pathologists who were blinded to clinical data, and histologic response was determined by comparison of pre- and post-treatment biopsies for severity of dysplasia. Complete histologic response was defined as a complete reversal of dysplasia to none dysplasia; partial histologic response was defined as an improvement in degree of maturation of epithelium; histologic stable disease was defined as no change in histology; histologic progressive disease was defined as any increase in severity grade.
Seven participants completed a 4-week cinobufacini intervention, and 8 participants completed a 12-week intervention. In all 15 participants, 2 participants experienced transient mild diarrhea, with one reporting abdominal pain and the other exhibiting hyperactive bowel sounds. These symptoms gradually alleviated as the medication continued, and no serious adverse effects were reported. It is worth mentioning that participant #4 suffered from primary thrombocytopenia, and the number of platelets returned to normal after the 4-week intervention. In the remaining 14 participants, there were no abnormalities in laboratory indicators.
The waterfall plot depicting changes in the lesion size is shown in Figure 1A. Although the lesion size decreased in 10 participants (66.7%), by size criteria (at least 50% size reduction in the lesion area), only participant #13, who received surgery for tongue cancer one and a half years ago, achieved complete clinical response after 12 weeks treatment, participant #2 and #14 achieved partial clinical response. No one had progressive disease. Of the 8 participants who took cinobufacini for 12 weeks, reduction in dysplasia from severe to none was observed in participant #14, reduction from moderate to none was observed in #15, reduction from mild to none was observed in #11 and #13. None of the participants had histologic progressive disease (shown in Figure 2). Except for three participants who initially reported being pain-free, 9 of 12 participants had varying degrees of pain reduction, and the NRS score showed a significant reduction from 3.23 ± 1.73 to 1.73 ± 0.92 (P < 0.05) (shown in Figure 1B).
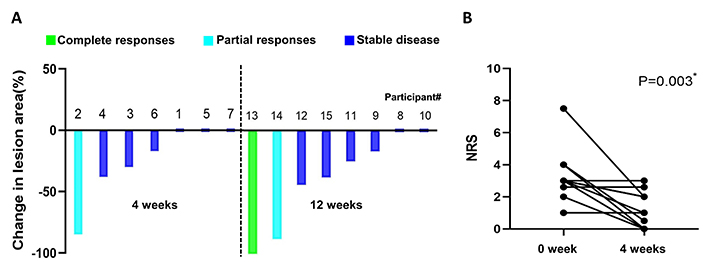
Clinical response of OPLs to cinobufacini. (A) The individual participant clinical response to treatment depicting the percentages of change in lesion size is indicated as a waterfall plot. (B) Individual change in NRS score from baseline to 4-week follow-up. OPLs: oral premalignant lesions; NRS: numerical rating scale
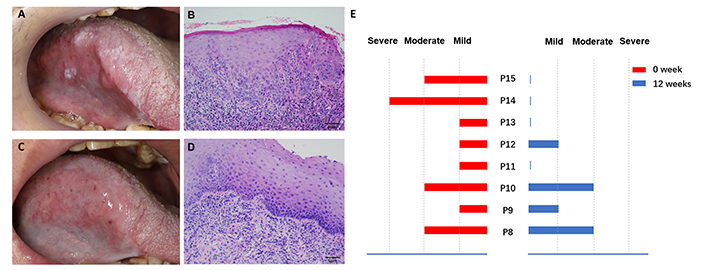
Histologic response of OPLs to cinobufacini. (A) Clinical appearance of participant #13 before cinobufacini treatment. (B) Baseline histologic appearance of participant #13 showing mild dysplasia. (C) Clinical appearance of participant #13 after 12 weeks of treatment. (D) Same patient showing a histologic response after 12 weeks of treatment. (E) Summary of the histologic responses of 8 participants evaluated. OPLs: oral premalignant lesions
Discussion
Here, we report the first clinical trial to investigate the effect of cinobufacini on patients with OPLs, the results demonstrated safety and tolerability of cinobufacini in all 15 participants, which is in line with previous reports [8, 14].
Of the eight patients who received 12-week treatment, one achieved complete clinical response, and 4 achieved complete histologic response, suggesting the potential therapeutic efficacy of cinobufacini in patients with OPLs. As the outcome was evaluated at two time intervals: short-term (4 weeks) and long-term (12 weeks), among the three positive clinical responders, two received 12-week treatment, it seems that a longer duration of time is feasible in the future larger trial.
Pain is a prevalent symptom among oral cancer patients, particularly when the lesion is located on the tongue, which often manifests before malignancy and serves as the initial cause for concern among patients [15]. Additionally, in patients with oral cancer, the degree of pain has been linked to a poorer prognosis [16]. As cinobufacini exhibits antinociceptive activity in previous studies [17], here, we also included pain assessment as an observational measure in OPLs treatment, and our results revealed a significant reduction in pain levels after the 4-week treatment period, showing the potential advantage of cinobufacini compared to other chemopreventive strategies for oral cancer.
This pilot study is the first to test cinobufacini treatment in patients with OPLs. Although no definitive conclusions can be drawn from this limited data, the significance of safety and efficacy of cinobufacini offers a promising therapy in the suffering of OPLs, and lays the foundation for larger trials.
Abbreviations
| NRS: | numerical rating scale |
| OPLs: | oral premalignant lesions |
Declarations
Author contributions
YL and PH: Investigation, Data curation, Formal analysis, Writing—original draft. JM, Yuhong W, Yuanyuan W, MW, YC, and JW: Investigation, Methodology. XW: Conceptualization, Funding acquisition, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study protocol was adhered to the principles of the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of the School of Stomatology, the Fourth Military Medical University (Ethical Approval number: IRB-YJ-2022005), and the Chinese Clinical Trial Registry (No. ChiCTR2300068529).
Consent to participate
The informed consent to participate in the study was obtained from all participants.
Consent to publication
The informed consent to publication was obtained from relevant participants.
Availability of data and materials
The datasets produced and/or analyzed in this study can be obtained from the corresponding author upon reasonable request.
Funding
This work is supported by grants from the National Clinical Research Center for Oral Disease of China [LCA202206]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.