Affiliation:
Pharmacy Department, Tishk International University, Erbil, Iraq
Email: javednaim88@rediffmail.com
ORCID: https://orcid.org/0000-0003-1558-0653
Explor Med. 2025;6:1001270 DOI: https://doi.org/10.37349/emed.2025.1001270
Received: September 02, 2024 Accepted: December 18, 2024 Published: January 10, 2025
Academic Editor: Luca Fiorillo, School of Dentistry University of Messina, Italy
The article belongs to the special issue Advances in Oral Cancer: Prevention, Diagnosis, and Therapeutics
Oral cancer is rare in Western nations but widespread in high-risk regions around the globe. Risk factors, such as tobacco usage, alcohol intake, and betel nut chewing, enhance the chance of the illness, making it mostly avoidable. Due to its high mortality, early detection is crucial. Prevention and diagnosis begin with oral mucosa lesions that may be malignant and local diseases that cause persistent inflammation. Clinical therapies for oral cancer mostly include surgery, radiation, and chemotherapy. Unsatisfactory therapeutic impact and harmful side effects remain clinical treatment’s key issues. Future research should examine the cancer microenvironment and treatment. This review examined oral cancer risk factors, preventative strategies, and early diagnostic approaches. This review also discusses immunotherapy methods for countering this fatal disease. Immunotherapy targeting the cancer microenvironment may provide a novel oral cancer treatment.
Cancer refers to uncontrolled cell proliferation that damages neighboring tissues. Oral cancer is the sixth most common cancer in the world. A person suffering from oral cancer may notice a little, inexplicable growth or sore in the mouth, cheeks, sinuses, tongue, hard or soft palate, or any portion of the mouth that extends from the base to the oropharynx. Data across the globe revealed that India accounts for one-third of the world’s total oral cancer burden and has the highest number of mouth cancer cases overall. According to Gupta et al. [1] countries going through economic transformation are more vulnerable to oral cancer. There has been a steady increase in the incidence of oral cancer with 90% being squamous cell carcinomas (SCC) [2]. The cancer cells in invasive SCC have progressed to more advanced stages of the oral cavity, as stated by Bishop et al. [3]. Lifestyle is one of the most significant factors that show a strong correlation with 75% of oral malignancies. These lifestyle factors or risk factors include smoking, chewing betel nuts or tobacco, drinking, not getting enough nutrition, using mouthwashes with excess alcohol, bad oral hygiene, having a weakened immune system, age, and gender.
In Southeast Asia, particularly on the Indian subcontinent, the most widespread habit is chewing betel quid with various additives. Betel quid, pan, or paan is a traditional Indian spice blend that typically includes betel leaf, areca nut, slaked lime, tobacco, and other herbs, and spices. Turmeric, cardamom, cloves, or aniseed in India are some of the other components commonly added to quid. In various parts of India, you can find these mixtures in the following forms: khaini (tobacco and lime), mishri (burned tobacco), zarda (boiled tobacco), gadakhu (tobacco and molasses), and mawa (tobacco, lime, and areca). In Central Asia and the Middle East, you can find nass (tobacco, ash, cotton, or sesame oil), and in Saudi Arabia and Sudan, you can find shammah (tobacco, ash, and lime). These products have been linked to an increased risk of oral cancer, according to research. Tobacco chewing is linked to oral cancer and precancerous conditions, including leukoplakia, erythroplakia, oral lichen planus (OLP), and oral submucous fibrosis, according to studies [4, 5]. Leukoplakia defines the presence of white patches of the oral mucosa (buccal mucosa, alveolar mucosa, palate, tongue, and floor of mouth) and doesn’t confirm the presence or absence of epithelial dysplasia. Its incidence varies between 1–3% with greater incidence in men [6, 7]. Erythroplakia (0.02–0.2% prevalence) with lesser frequency appears as red lesions with a velvet texture on the oral mucosa. It is asymptomatic in nature but shows a burning sensation or soreness while eating [8, 9]. OLP is an immunologically driven chronic inflammatory condition affecting the oral mucosa of middle-aged patients. Erosive OLP reflects the highest risk of malignant transformation [10, 11]. Submucosal fibrosis represents chronic fibrotic lesions with malignant potential (transformation rate is 9%). These fibrotic lesions result in loss of elasticity in the affected oral mucosa tissue, thereby limiting mouth opening [12]. Furthermore, betel quid chewing is also responsible for higher incidences of cardiovascular diseases along with a proportionate increase in obesity, metabolic syndrome, diabetes, and chronic kidney disease, thereby worsening the condition of the patients [13–17]. Recent findings from the International Agency for Research on Cancer suggest that smokeless tobacco may contribute to the development of oral cancer [18]. Malki et al. [19] and Oji and Chukwuneke [20] include hereditary variables, mate drinking, and chronic trauma as additional risk factors (Figure 1). But if someone smokes and drinks simultaneously, the consequences can be disastrous [21]. Oral cancer, which mostly affects males, accounts for around 25% of all anticipated cancer cases in these nations (low and middle-income countries) [22]. Buccal mucosa accounts for about 32% of oral cancer cases, tongue for 22%, lower lip for 11%, palate for 11%, vestibule for 8%, alveolus for 5%, floor of the mouth for 5%, and gingiva for 3% [23]. There are about 4,000 compounds in tobacco smoke, including 60 known carcinogens such as nicotine, arsenic, methanol, and methoxy methyl furfural [24]. Activation of procarcinogens is accelerated by alcohol, and it also acts as a solvent to allow dangerous carcinogens to enter cells in the body. As soon as the tumor reaches a certain size, localized discomfort begins to manifest, prompting the pursuit of medical intervention.
Oral cancer is difficult to diagnose in its early stages since the disease often causes no symptoms at all. A higher percentage of patient survival may be achieved with an earlier diagnosis. When caught early, the survival chances are about 80–90% [30]. Death rates rise as a result of detection delays. There are several therapeutic options available, but the survival rate is still dismal.
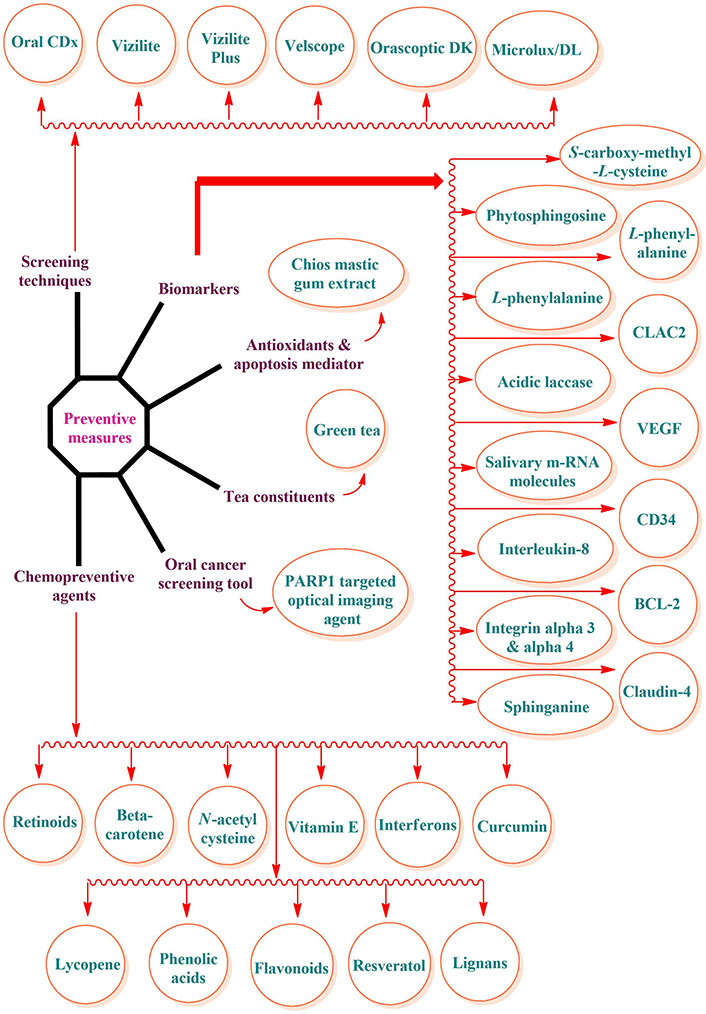
Early identification and prediction of oral cancer may be achieved with the use of screening technologies that aid in the detection and localization of oral cancer [31]. Early diagnosis, monitoring, and differentiation of oral cancer can be aided by salivary biomarkers such as L-phenylalanine, angiogenetic markers such as cluster of differentiation factor 34 (CD34), genomic biomarkers like integrin α3 and integrin β4, and the cloning of an acidic laccase gene 2 (proteomic biomarker) [32] (Figure 2).
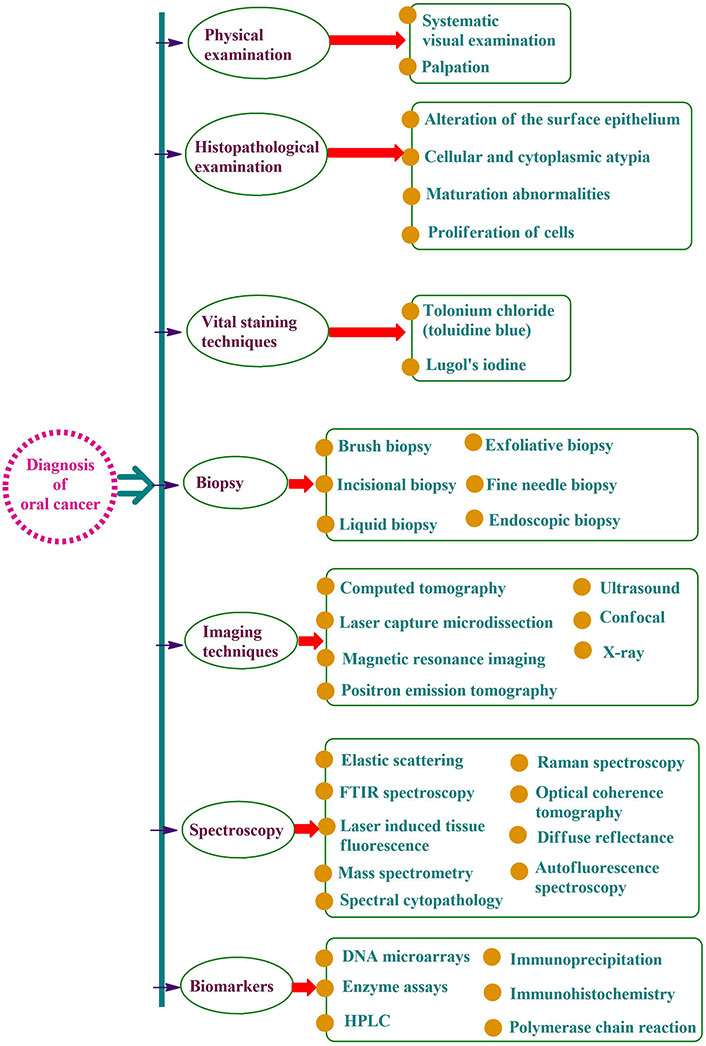
Oral cancer is a life-threatening condition that has a significant impact on the quality of life when detected at later stages. Oral cancer screening programs play an important role in the early identification of oral cancer, leading to prompt diagnosis followed by treatment in the due course of time. The screening is a non-invasive simple procedure involving inspection of the oral mucosa for any unacceptable changes that may worsen leading to oral cancer. As per the World Health Organization, focus must be given to measures to ensure that prevention is an integral part of the national oral cancer control program. The effectiveness of these screening programs relies on:
Early detection: oral cancer can be overlooked in its early stages of occurrence as the symptoms may be very subtle or minor. The screening programs can assist us in the identification of early signs of oral cancer such as lumps, and sores in the oral mucosa, permitting better treatment results [48].
Improved prognosis: on detection of oral cancer in its early stages, the possibility of treatment success, long-term survival, and improvement in the quality of life of a patient will be higher. The screening programs enable us to detect oral cancer before its progression to advanced stages.
Increases awareness: the screening programs help to raise awareness regarding various risk factors and warning signs of oral cancer. This encourages individuals to perform regular dental visits to keep a check on oral mucosal health for any irregularities.
Cost-effective: early detection with the advent of screening programs may help bring down the healthcare expenses for complex treatment procedures [49].
Therefore, reducing and controlling mortality due to oral cancer should be the primary focus of prevention efforts. There are three levels of preventative actions: primary, secondary, and tertiary. Raising public knowledge of risk factors and encouraging behavioral change are the main objectives of primary preventive measures. To combat oral cancer, it is crucial to raise awareness of the illness’s terrible consequences, clarify to the public that the condition is curable, and disseminate information about the benefits of useful screening procedures [50]. Figure 3 provides a concise summary of the steps to take to avoid oral cancer. Retinoids, β-carotene, N-acetyl cysteine, non-steroidal anti-inflammatory drugs (NSAIDs), vitamin E, retinoids, and curcumin are beneficial in the chemoprevention of oral cancer (Figure 3) [51, 52]. The pineal gland secretes melatonin, which is important because it protects against oral cancer, has antioxidant properties, and causes oncostatic effects [53]. Inducing apoptosis and limiting cell proliferation, the ingredients of tea (camellia sinensis) suppress carcinogenesis of the oral cavity, lung, esophagus, etc. [54–56].
Many different approaches exist for the treatment of oral cancer. The standard treatments for cancer at the moment include surgical procedures, radiation therapy, and chemotherapy [57–59]. Some individuals with tongue cancer may have significant difficulties eating and speaking after surgery, which is often determined by the cancer size. Radioactive skin injury, radioactive mucous membrane injury, and radioactive stomatitis are all possible side effects of radiation that might affect the local skin and mucous membranes [60, 61]. Systemic treatments like chemotherapy might cause side effects such as pancytopenia and gastrointestinal distress [62]. When alternative treatments for oral cancer generate difficulties, traditional Chinese medicine is often the first line of defense. Despite this, the oral cancer microenvironment is complicated, and as a result, there are few safe and effective therapeutic options. A thorough comprehension of the makeup and features of the cancer microenvironment is the foundation for creating immunotherapy, and there are intricate networks of interactions between the various parts of the cancer microenvironment. Prospects for immunotherapy research in the treatment of oral cancer will be explored to improve the quality of life among oral cancer patients [63, 64]. This review highlights various risk factors responsible for oral cancer with various diagnosis procedures and preventive measures to be taken to deal with. A better understanding of oral cancer will pave the way for immunotherapy to be employed as a significant treatment option.
From first exposure to risk factors to clinical identification of a lesion, there are many stages in the development of oral cancer. People who smoke may be exposing themselves to carcinogens. Additional research has shown that tobacco smoke extract may activate the epidermal growth factor receptor (EGFR) in a controlled laboratory setting. This activation, in turn, leads to an increase in the production of prostaglandins, such as prostaglandin E2 (PGE2), which might potentially boost EGFR signal transduction even more. Increased cyclin-D1 activity is a downstream process initiated by EGFR activation, and cyclin-D1 overexpression is common in head and neck cancer [65]. Chromosome 3p and 9p deletions or mutations are genetic abnormalities that are present early in carcinogenesis. In the early stages of carcinogenesis, telomerase activation also takes place. Typically, deletions or mutations on chromosome 17p [which affects the p53 tumor suppressor gene (TSG)], chromosome 13q, and chromosome 18q manifest later on. A different molecular process may be at work in individuals whose tumors contain human papilloma virus (HPV) mRNA, as the frequency of chromosome 3p, 9p, and 17p deletions is much lower in these patients. One possible mechanism of HPV-mediated carcinogenesis is that the viral proteins E6 and E7 deactivate p53 and retinoblastoma (Rb) protein, leading to cell cycle dysregulation [66].
According to research, oral cancers begin as a premalignant progenitor cell line and then undergo a process known as clonal expansion to progress to more advanced stages. As mutations and abnormalities in deoxyribose nucleic acid (DNA) accumulate, clonal proliferation continues until cancer develops into a full-blown disease [67]. Two main genetic changes are the inactivation of TSGs and the activation of proto-oncogenes, which play a crucial role in the development of oral cancer. When normal mucosa transforms into premalignant mucosa/lesions, changes take place during early mutagenesis. Research has linked the development of SCC to a range of premalignant lesions such as leukoplakia, erythroplakia, OLP, and oral submucous fibrosis are prevalent and may develop into malignancy in several ways. According to the World Health Organization (2005), premalignant lesions may be categorized as mild, moderate, severe, or carcinoma in situ based on the degree of dysplasia [68, 69]. Seventy to eighty percent of oral cancers have chromosomal region 9p21 lost. Research has connected viral infections to the development of cancers of the mouth and throat. HPV is the most prevalent virus linked to the development of oral cancers. The majority of oral SCCs are caused by HPV 16 and HPV 18. Unlike those who misuse smoke or alcohol, the process of HPV mutagenesis seems to be different. HPV viral oncoproteins may destroy TSGs instead of altering them. It is believed that the genetic and cellular alterations are induced by the two early gene proteins encoded by HPV 16 and 18, E6 and E7. Cellular division is halted when E6 inactivates or degrades p53 [70]. Another mechanism by which E7 messes with cell cycle control is its alleged role in Rb degradation. Some research suggests that “traditional” carcinomas and HPV-mediated oropharyngeal and oral cancers are not the same. There may be cellular alterations connected to HPV that are associated with verrucous cancer and proliferative verrucous hyperplasia. Cancer patients who test positive for HPV tend to be younger, never smoke, have metastases to lymph nodes at an earlier stage, and have a history of more frequent heterosexual and orogenital sexual interactions in their social lives. Contrasted with oral cavity lesions, which only revealed HPV DNA in 12% to 18% of cases, oropharyngeal cancers, and tonsillar cancer in particular, had the greatest rates of HPV DNA detection, ranging from 45% to 67% of individuals tested [71].
Despite treatment, people with oral SCC (OSCC) still have a bad prognosis. According to Inchingolo et al. [28] and Mehrotra and Gupta [72], the key to increasing patients’ survival rates is early diagnosis and treatment. There is a dearth of succinct information on the numerous specific and non-specific methods that have been developed over the years to anticipate the malignant progression of oral cancers and to address new treatment approaches (precision medicine). Additionally, there is still some doubt about their usage in regular clinical practice [73, 74]. Numerous novel, as-yet-untested biomarkers and treatments have emerged as promising alternatives to conventional ones, owing to the ongoing investigation into genomics, translational medicine, biochemistry, and molecular biology [75–77]. Whether the illness is in its early stages, has progressed locally, or is recurring, surgery is an important tool. Fortunately, surgical knowledge expanded to include head and neck cancer due to progress in anatomy. From antiquity until the nineteenth century, all surgical procedures posed serious risks to patients due to a lack of anesthesia, antiseptic techniques, airway control, and the possibility of bleeding. In the twentieth century, researchers investigated the use of chemotherapy and radiation for the treatment of oral cancer [78]. Surgery, radiation, chemotherapy (5-fluorouracil, carboplatin or cisplatin, Taxol-based therapies, and irinotecan in the most advanced cases), and, more recently, monoclonal antibodies (mAbs) targeted against the EGFR are often part of a multidisciplinary approach for patients with locally advanced oral cancers. Oral malignancies often activate the phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway, which may be a therapeutic target due to its cancer-promoting function. Clinical studies examining the effects of direct PI3K and/or mTOR inhibitors in the mouth and metformin, which inhibits mTOR indirectly, are both looking at the dependency of this pathway on oral cavity tumors in individuals with possible premalignant lesions [79–85].
Because of its well-established efficacy in preventing and treating mandibular osteoradionecrosis, preliminary data suggests that adding hyperbaric oxygen (HBO2) therapy to the chemo-radiation standard of care is a safe, feasible, and well-tolerated option [86]. And lastly, T-cell exhaustion is an important new mechanism of tumor immunosuppression. Derived from inflammatory monocytes, tumor-associated macrophages (TAMs) are essential regulators of tumor growth. Typically, TAMs encourage tumor growth while reducing the immune response via innate and adaptive immunological pathways. Nevertheless, TAMs still can limit tumor growth via pro-inflammatory mechanisms, which is a double-edged sword. Promising preclinical and clinical results have been seen from treatment techniques targeting TAMs, which either deplete the immunosuppressive function or elicit anti-tumor capacity. It is now possible to reactivate anticancer T-cell responses using macrophage-centered treatment methods such as anti-programmed death 1 (PD-1) T-cell [87].
Metformin has appeared as an excellent antidiabetic medication with the potential to treat and prevent cancer of the oral mucosa, particularly OSCC. Its varied mechanism involves inhibition of cell proliferation, induction of apoptosis, and modulation of principle oncogenic pathways, making it a potential and valuable molecule for cancer therapy [88]. A pivotal study was done in 2020 to characterize the relationship between metformin and oral cancer, which resulted in a negative association between them, thereby revealing that people on metformin therapy have a lower risk of developing oral cancer [89]. Metformin has revealed varied effects on oral SCC and influenced many cellular mechanisms to inhibit the advancement of oral cancer [90]. Inhibition of cell proliferation is the principal mechanism via which metformin acts on cancerous cells [91]. It restricts the proteolysis of the nerve growth factor receptor (NGFR), thus bringing down the generation of its intracellular domain NGFR-N (inhibits p53 to promote cell proliferation). As a result, metformin was found to reverse the suppressive effect of NGFR-N on p53 (tumor suppressive protein) to inhibit oral squamous cancer cell proliferation [92, 93]. Preclinical data reflected that metformin successfully reverses the epithelial-to-mesenchymal transition (EMT), which was found to be induced by CoCl2 in OSCC cells. Metformin causes the downregulation of principal signaling pathways that cause the regulation of EMT including mTOR, hypoxia inducible factor-1α (HIF-1α), pyruvate kinase M2 (PKM2), and signal transducer and activator of transcription 3 (STAT3) [94, 95]. Inhibition of these pathways by metformin resulted in reduced cell proliferation, migration, and invasion to bring down the metastatic potential of OSCC cells. Metformin was also found to act on OSCC cells via organic cation transporter 3 (OCT3), which is widely expressed in head and neck SCC (HNSCC) and premalignant lesions (OLP). Preclinical data revealed that metformin may modulate immune responses by intensifying tumor-infiltrating T-cell functionality and that can be identified via several immune markers such as CD8, CD163, and PD ligand 1 (PD-L1) [96, 97]. Metformin has also highlighted its potential to be effective against ovarian cancer, breast cancer, colorectal cancer, thyroid cancer, gastric cancer, esophageal cancer, cervical cancer, nasopharyngeal cancer, hepatocellular carcinoma, bladder cancer, and prostate cancer [98–107]. It affects the tumor microenvironment by reducing glucose supply and limiting nutrient availability, thereby making it difficult for the proliferation of cancerous cells [108, 109]. Metformin also showed its potential in preventing and reducing the risk of gingival/periodontal disease [110].
A novel approach to oral cancer treatment, immunotherapy enhances the targeted immune response to cancer by using biotechnology and immunological approaches. Because of its groundbreaking nature and remarkable effectiveness, cancer immunotherapy was named 2013’s most significant scientific discovery. Emmanuelle Charpentier, a French microbiologist, and Jennifer Doudna, an American biologist, were awarded the Nobel Prize in Chemistry in 2020 for their “development of genome editing methods”. Various forms of immunotherapy, such as adoptive cell immunotherapy (ACI), antibody-based therapy, cytokine therapy, tumor vaccines, gene therapy, and others, have great potential for use in the treatment of cancer [111–113].
Produced by a single B-cell clone, mAbs bind to a particular epitope and are very homogenous antibodies. Multiple mAb-based oral cancer therapy trials are now underway. The nine ongoing clinical studies include five immune checkpoint inhibitors and four mAbs against angiogenesis (Figure 4). Also, nucleophosmin, c-Met protein with tyrosine kinase activity, and receptor tyrosine-protein kinase erbB-3 are other significant targets for therapeutic therapy of oral cancer using mAbs. Additionally, there is promising evidence that mAbs may help prevent oral cancer. A precancerous lesion of oral cancer, oral proliferative verrucous leukoplakia (OPVL) is an extremely uncommon form of refractory leukoplakia. The progression to verrucous or SCC of the mouth is probable. Nivolumab is now being tested in a clinical study for the prevention of OPVL and has shown promise in shrinking the white lesions in the mouth of participants as well as reducing their risk of cancer (NCT03692325) [114–116].
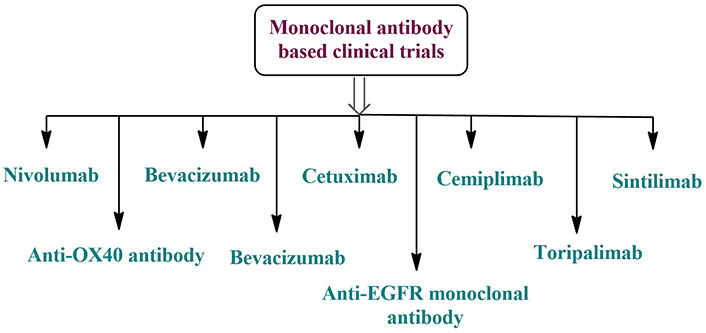
Monoclonal antibody-based clinical trials for the treatment of oral cancer [117]. EGFR: epidermal growth factor receptor
ACI may be administered to cancer patients either as a stand-alone treatment or, more crucially, as an adjuvant to conventional cancer treatments such as chemotherapy, radiation, and surgery. At present, lymphokine-activated killer (LAK) cells, chimeric antigen receptor T (CART) cells, anti-CD3 antibody induced activated killer (CD3AK) cells, cytokine-induced killer (CIK) cells, and dendritic cells (DCs) are the primary ACI treatment cells used in clinical research and applications [118].
Stimulated immune cells often release cytokines, which are quite effective. They can boost the killing impact of immune cells by directly stimulating stromal cells and immune effector cells at the tumor site. Eight clinical trials with mAbs in oral malignancies indicated four interferon-α (IFN-α), three interleukins, and one salivary cytokine (Figure 5). Additional variables will be included in cancer immunotherapy as cytokine research progresses [117, 119].
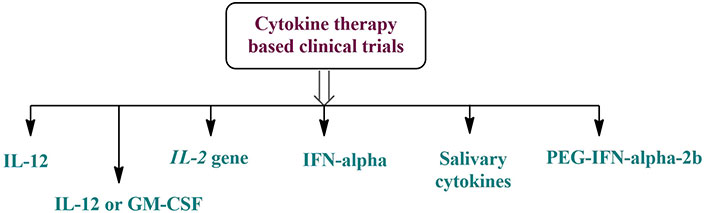
Cytokine therapy-based clinical trials for the treatment of oral cancer. GM-CSF: granulocyte-macrophage colony-stimulating factor; IFN-α: interferon-α; PEG: polyethylene glycol
One targeted, risk-free, and well-tolerated method of cancer therapy is the tumor vaccination. Tumour vaccines may be broadly categorized into three primary types: inactivated, protein/polypeptide, and nucleic acid-based. About the keywords (“oropharyngeal cancer” OR “oral cancer” OR “oral cavity cancer”) and vaccine, there are several active investigations concerning cancer vaccination in oral malignancies. Figure 6 shows that out of the 17 clinical studies, 10 are for vaccines based on HPV antigens, 4 on proteins or polypeptides, 2 on vaccines mixed with mAbs, and 1 on DCs. Oral cancer treatments mainly include HPV antigen-related vaccinations and combination therapy. Furthermore, the future of oral cancer therapy may lie in the development of nucleic acid vaccines and neoantigens [120].
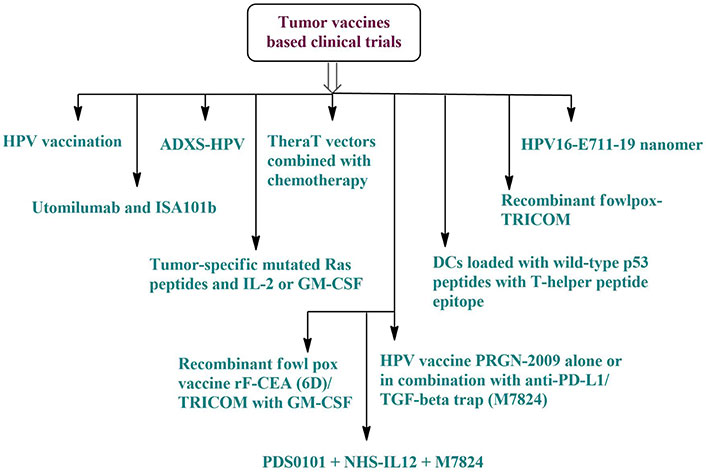
Tumor vaccine-based clinical trials for the treatment of oral cancer. ADXS: axalimogene filolisbac; DCs: dendritic cells; GM-CSF: granulocyte-macrophage colony-stimulating factor; HPV: human papilloma virus; rF-CEA: recombinant fowlpox carcinoembryonic antigen; TGF-β: transforming growth factor-β; TRICOM: triad of costimulatory molecules
Therapeutic genes, nucleic acid vaccines, RNA interference, and gene probe technologies are all examples of gene therapy that may be controlled at the nucleic acid level to cure cancer [121, 122]. Additionally, genes involved in interactions with the immune system may be targeted using gene therapy. Furthermore, future research should focus on developing gene therapy techniques that target genes connected to the immune system that are associated with oral cancer. Several gene-based immunotherapy trials for oral malignancies are now active [123, 124]. Five therapeutic studies using cytokines, CART, and gene vaccines are shown in Figure 7. As molecular and cellular biology as well as clinical medical technology continue to advance, gene therapy is poised to become a standard approach to treating cancer in the future and play a pivotal role in beating refractory cancers [125].
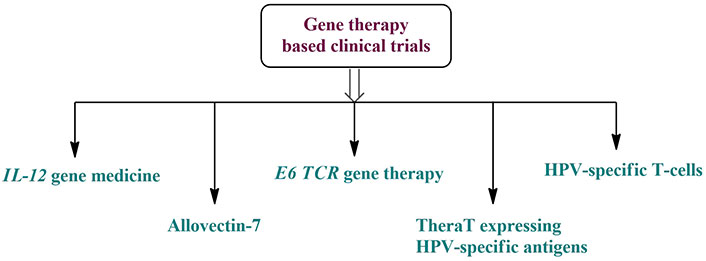
Gene therapy-based clinical trials for the treatment of oral cancer. HPV: human papilloma virus; TCR: T-cell receptor
Extensive patient genetic testing for facilitating targeted, personalized treatments is expected to be a part of the future of oral cancer management, as is the application of stem cell technologies to “grow” suitable organs that could replace missing tissue without the need for immunosuppression [126, 127]. Unfortunately, the prognosis for patients diagnosed with oral cancer at stages III and IV has not changed much over the past several decades, even though chemotherapy, radiotherapy, and surgical resection have improved the quality of life for patients. Whether the disease is in its early stages, has progressed locally, or is recurring, surgery is an important tool. The most effective method for treating oral cancer is a thorough neck dissection, and the presence of lymph nodes in the patient’s body is still a major indicator of prognosis. Recent advances in treating oral cancer have included mAbs directed against the EGFR [28, 128, 129].
Oral cancer development may be influenced by multiple risk factors with a higher inclination towards cigarette smoking and alcohol intake. Another important factor is inflammation, which is thought to be influenced by genetic predisposition. The causes, symptoms, and methods of early identification and treatment of oral cancer have been the subject of a great deal of research. Oral cancer is a serious illness that requires better prevention and treatment methods because of its high death and morbidity rates. Patients diagnosed with oral cancer typically have surgery as a primary method of treatment followed by radiation and chemotherapy. Mental and physical health problems are associated with all of the aforementioned therapeutic approaches. Preventing and detecting oral cancer at an early stage can lessen the severity of these dire consequences. Although immunotherapy has been examined for its potential use in treating oral cancer, more research is needed to determine its efficacy in preventing and treating oral cancer. Moreover, early detection and prevention of oral cancer are both enhanced by a deeper knowledge of the molecular pathways involved in its development. The identification of ways to minimize the morbidity and fatality rates of oral cancer requires substantial effort.
ACI: adoptive cell immunotherapy
CART: chimeric antigen receptor T
CD34: cluster of differentiation factor 34
DCs: dendritic cells
DNA: deoxyribose nucleic acid
EGFR: epidermal growth factor receptor
EMT: epithelial-to-mesenchymal transition
HPV: human papilloma virus
mAbs: monoclonal antibodies
mTOR: mammalian target of rapamycin
NGFR: nerve growth factor receptor
OLP: oral lichen planus
OPVL: oral proliferative verrucous leukoplakia
OSCC: oral squamous cell carcinoma
PD-1: programmed death 1
PI3K: phosphatidylinositol 3-kinase
Rb: retinoblastoma
SCC: squamous cell carcinoma
TAMs: tumor-associated macrophages
TSG: tumor suppressor gene
MJN: Conceptualization, Data curation, Methodology, Supervision, Resources, Writing—original draft, Writing—review & editing.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4305
Download: 57
Times Cited: 0
Yang Liu ... Xinwen Wang
Chie Ching Tan ... Spoorthi Ravi Banavar
Supraja Salwaji ... Giuseppe Minervini
