Affiliation:
Department of Biotechnology, Institute of Applied Science and Humanities, GLA University, Mathura 281406, Uttar Pradesh, India
Email: sarmistha_pharmacol@yahoo.com
ORCID: https://orcid.org/0000-0001-5324-1957
Affiliation:
Department of Biotechnology, Institute of Applied Science and Humanities, GLA University, Mathura 281406, Uttar Pradesh, India
Affiliation:
Department of Biotechnology, Institute of Applied Science and Humanities, GLA University, Mathura 281406, Uttar Pradesh, India
Affiliation:
Department of Biotechnology, Institute of Applied Science and Humanities, GLA University, Mathura 281406, Uttar Pradesh, India
ORCID: https://orcid.org/0000-0002-7383-188X
Explor Med. 2025;6:1001272 DOI: https://doi.org/10.37349/emed.2025.1001272
Received: September 26, 2024 Accepted: December 06, 2024 Published: January 13, 2025
Academic Editor: Jingchao Li, Donghua University, China
According to research, hepatocellular carcinoma (HCC), ranks third globally in terms of cause of death and is the fifth most common type of cancer overall. Finding novel means of diagnosis and treatment is therefore crucial. The use of nanotechnology as a cancer treatment has drawn a lot of interest recently. Despite significant advancements in detection and treatment, there is still a long way to go before this disease is completely eradicated. Therefore, it’s critical to find innovative ways to diagnose and cure conditions. In particular, the substantial inertness of metallic nanoparticles (NPs) and their nanoscale structures, which have sizes comparable to many biological molecules, attract a great deal of interest in the biomedical field. Due to their exceptional optical qualities, chemically modified surface through the attachment of various ligands, biocompatibility (bio-inertness and low cytotoxicity), and superior optical properties, gold NPs (AuNPs) have garnered significant interest. The current review discusses the efficiency of AuNPs in various fields, including imaging, immunotherapy, and photothermal therapy for treating liver cancer. Finally, this review summarized the limitations of the prospects of the AuNPs.
One of the main causes of death globally is cancer, and over the next 20 years, there will likely be 22 million new cases of the disease, partly as a result of the aging population [1]. The World Health Organization (WHO) reports that cancer was the world’s biggest cause of death in 2007 with 7.9 million deaths. Therefore, to develop the most efficient methods of cancer detection, monitoring, and therapy, the boundaries of cancer research are always being tested. The discoveries that occurred in cancer research would undoubtedly help humanity and save many lives. These days, chemotherapy, radiation therapy, and surgery are the primary therapeutic modalities utilized to treat cancer. These treatments can be given singly or in different combinations [2].
Medications that have a cytotoxic impact and interfere with the processes that allow cancer cells to proliferate quickly are given as chemotherapy treatments [3–5]. Because of the partially non-selective uptake of the chemotherapeutics into both healthy and malignant cells in tissues and organs, conventional chemotherapy is recognized for its severe side effects despite its effectiveness. The development of nanomedicine has led to significant advancements in recent times, offering chemotherapy a novel and valuable supplement [6, 7]. Chemotherapeutic medications kill not only cancer cells but also healthy cells such as the immune system and bone marrow cells by specifically targeting fast-dividing cells [8]. The patient’s body experiences extensive “collateral damage” as a result. To kill tumor cells, radiation therapy uses high-intensity radiation, such as X-rays and gamma rays, which invariably hurts healthy tissues nearby [9].
Hepatocellular carcinoma (HCC), another name for liver cancer, is a prevalent malignancy [9], yet nearly all of its patients pass away within a year due to its extremely high fatality rate. Hepatitis virus infection was the cause of the elevated death rate. However, there is currently no effective radiosensitizer available for therapeutic use, and liver cancer cells are not radiation-sensitive. Therefore, developing a safe and effective radiation therapy for hepatocellular cancer is of utmost importance. Thus, it is critical to develop an effective and safe method for HCC radiation therapy.
Targeting therapeutic chemicals to specifically target tumor cells while sparing healthy tissues from injury is a vital step toward improving cancer therapy, given the inadequacies of current cancer treatment approaches. One of the newest areas of interest in nanotechnology research is this. The creation of materials with nanoscale dimensions ranging from 1 nm to 100 nm is referred to as nanotechnology [10]. These nanoparticles (NPs) are special because of their small size, which gives them different chemical and physical characteristics from their bulk counterparts [11]. Research on nanomaterials is growing at a rapid pace, which bodes well for the development of new diagnostic techniques and treatments for human diseases in the future [12]. The National Institutes of Health in the United States has dubbed this area of nanotechnology used in illness detection, monitoring, and therapy “nanomedicine” [10].
This review will concentrate on gold NPs (AuNPs) and their potential as tumor sensors, drug delivery agents, and enhancers in plasmonic photothermal treatment for the eradication of malignancies, among the various nanomaterials being explored for nanomedicine applications [13]. Scientists and technicians find AuNPs, also known as AuNPs, to be particularly appealing among these NPs. AuNPs come in both spherical and non-spherical shapes, including tetrahedral, sub-octahedral, octahedral, decahedral, icosahedral multiple twined, multiple twined, irregular shapes, and nanorods [14, 15]. Metal NPs are created via a variety of chemically, physically, and biologically synthesized methods, and they are typically classified into two groups: top-down and bottom-up [15–17]. While many techniques exist for the simple synthesis of pure AuNPs, the chemical reduction of a metal salt in the presence of a stabilizing agent is the most standard way [18–20]. Because of their distinct physicochemical and optical characteristics, AuNPs are employed in a variety of domains in contemporary medical and biological research. These days, there is a vast array of uses for these particles, including pathology and disease detection and therapy [21], wound healing [22], genomics [23], immunoassays [24], optical equipment [25], and electronic [26]. As AuNPs have outstanding optical qualities and may be chemically changed by attaching various ligands with low cytotoxicity and bio-inertness, they are promising prospects in the field of cancer nanotechnology [27]. The use of AuNPs is growing in popularity across a number of academic disciplines for a number of reasons. To begin, AuNPs are believed to be relatively biologically non-reactive and so suitable for in vivo applications in contrast to the very toxic cadmium and silver NPs [28]. Strong optical characteristics are provided by localized surface plasmon resonance (LSPR) in AuNPs [29], surface chemistry is easily programmable, allowing for a variety of surface functional group additions [30], and particle size and shape may be easily controlled during synthesis [31, 32]. Additionally, other functionalized nanomaterials based on fullerenes and magnetic NPs also proved to be beneficial for medical diagnostics and therapy [33–37].
AuNPs can be synthesized in several ways, both chemically and physically. To be more precise, there are two types of these manufacturing methods: top-down and bottom-up [38]. The production of homogenous AuNPs with diameters ranging from 5 nm to 40 nm using the radiation technique was reported by Tue Anh et al. [39]. The outcomes demonstrated that producing AuNPs with a controlled size and high purity may be accomplished by the irradiation technique. It has been documented that AuNPs can be synthesized using the sonochemical approach, which is quick, simple, affordable, appealing, and environmentally benign. Fuentes-García et al. [40] used varying ultrasonic irradiations (60 W, 150 W, and 210 W) to synthesize AuNPs. After 60 min of radiation exposure, colloidal AuNP solutions were obtained from gold acid (HAuCl4) and sodium citrate [40]. The Brust and Turkevich strategies are the most widely used bottom-up approaches for producing AuNPs. To produce uniform, spherical AuNPs that range in size from 10 nm to 20 nm, the Turkevich method relies on decreasing metallic ions [41]. Sodium citrate is typically employed to create a colloidal dispersion that inhibits particle aggregation in addition to its role as a reducing agent and stabilizer [42]. Moreover, amino acids, ascorbic acid, and UV radiation can be utilized in place of citrate [43]. First published in 1994 [41], the Schiffrin–Brust technique is based on many procedures that are beneficial for the synthesis of AuNPs in an organic system with excellent stability. Particle sizes as small as 2 nm can be obtained with this technique, which uses tetrabutylammonium bromide as a transfer agent from organic to inorganic solutions [44]. In addition to these methods, the “growing seed” process is frequently employed to create AuNPs in other shapes, including nanocubes and nanorods [45, 46]. The Au ion ratio, which can be anywhere between 5 nm and 40 nm [47], determines the size of the particles that are created.
Another key aspect here is the size and shape of the NPs which influences the therapeutic and other applications. Different-sized AuNPs, ranging from 10 nm to 50 nm, were tested on human dendritic cells (DCs) due to their significant potential in biomedical applications for cancer diagnostics. The findings suggested that the effect was notably more pronounced with the use of AuNPs measuring 10 nm, which inhibited the production of IL-12 p70 in DCs when exposed to lipopolysaccharide [47]. Research indicates that small-sized NPs (10–15 nm) are the most effective at controlling cancer cells among the three different sizes studied. Their tiny size allows them to easily enter cells and interact with organelles [48].
Strong surface area-to-volume ratios of metal NPs allow for easy bioconjugation with therapeutic compounds and/or ligand targeting to a cancer location. The enhanced permeability and retention (EPR) effect of these substances allows them to passively collect within tumor locations and pass through physiological barriers [49, 50]. Theranostic NPs are those that can function as agents with both therapeutic and diagnostic capabilities at the same time. LSPR, an optical phenomenon, is the basis for the majority of AuNPs used in nanomedicine [51]. SPR is a synchronized oscillation in the conduction-band electrons that can occur when a metal particle is exposed to electromagnetic waves of a certain wavelength caused by a dielectric [52]. Gold is a common metal with assignable SPR bands. Mie theory states that the dielectric constant of the surrounding medium, as well as the particle size, shape, structure, and composition, all affect the LSPR band location of the AuNPs. AuNPs can provide tunable absorption in the visible and near-infrared (NIR) wavelength ranges by adjusting these parameters. Light can penetrate deeply into soft tissues when the long photoperiod (LSPR) band shifts to the NIR region in the 700–900 nm range, sometimes known as the “transparent window”. This makes the light useful for in vivo medical applications, such as the detection, diagnosis, and therapy of malignant cells [53, 54]. We covered recent developments in the use of AuNPs for various liver cancer nanotechnology applications in this review.
The superior optical property known as SPR of AuNPs, like that of many valuable metals, enables their use in NIR resonant medical imaging modalities, such as computed tomography (CT) [54], X-ray scatter imaging [55], fluorescence imaging [56], photoacoustic imaging (PAI) [57], and magnetic resonance imaging (MRI) [58]. AuNPs also have low toxicity and are nonimmunogenic. Since the processes for creating AuNPs are simple, it is possible to control their size, shape, and surface modification. All of these characteristics suggest that AuNPs can be tailored in different ways for controlled and targeted medication administration as well as for the localized hyperthermia of cancer tissue [59]. Based on all these features, it appears that AuNPs can be customized in various ways for both localized cancer tissue hyperthermia and targeted and regulated drug delivery [60]. Size is one of the key components of AuNPs that affects the body’s circulation half-life, systemic toxicity, tumor formation, and other elements crucial for therapeutic and diagnostic applications. It is crucial to comprehend the underlying effects of AuNPs of various sizes as the number of applications for AuNPs increases. The fluorescence of AuNPs is influenced by a variety of parameters, such as surface chemistry, oxidation state, size, and surrounding environment.
Radiation therapy for cancer is intimately linked to AuNPs’ capacity for cellular absorption, which is influenced by both particle size and surface chemistry [61–65]. The high zeta potential of bare AuNPs makes them known to be unstable and likely to congregate in blood [66]. It has been shown that a surface-stabilized coating increases the cellular absorption and stability of AuNPs in blood [67–69]. For instance, it has been observed that bare AuNPs generated 150 nm particles that were taken up via micropinocytosis [70]. On the other hand, AuNPs coated with polyethylene glycol (PEG) were endocytosed and exhibited a relatively steady absorption rate [71–73].
AuNPs for molecular imaging in cells and biological systems were compiled by Bouché et al. [74], while applications of AuNP aggregation were covered in studies conducted by Alizadeh and Nazari [75]. The synthetic approaches and latest developments in fluorescence sensing using AuNPs were reported by Halawa et al. [76]. The processes involved in creating monodisperse AuNPs and their potential therapeutic uses were not the subject of any of this research.
One of the basic core characteristics of AuNPs is SPR, which is mostly dictated by their size and form [77]. Plasmon occurs when specific light wavelengths interact with the material interface’s conduction band, resulting in a dipole oscillation that depends on the incident light’s ionic lattice and electromagnetic field. Plasmon is a collective oscillation of the free electrons at the material interface. SPR is the name for the maximum oscillation that occurs at a specific light frequency [78]. Depending on their size, AuNPs can absorb light very intensely, and the SPR band extends from the visible to the infrared spectrum.
SPR also provides surface plasmon scattering, which is the result of light striking AuNPs and producing electron oscillation, which reemits photons at the same wavelength due to photon energy. The wavelength can be used for imaging and diagnosing different diseases, including lung, prostate, and breast cancers. This is because changing the material’s interface with different receptors and the ensuing interaction with different structures, including cells, affects the wavelength [79]. The reduction of bulk gold size to nanoscale dimensions results in a rise in the surface-to-volume ratio, which influences the surface energy of AuNPs and enhances atom alignment on the NP surface. Therefore, to improve their biocompatibility, AuNPs can interact with a wide variety of substances [80].
AuNPs must have their surfaces modified in order to increase biocompatibility and lessen toxicity brought on by surfactants. The key method for functionalizing AuNPs is the thiol gold reaction, which depends on the strong affinity between Au and thiols. By replacing the cetyl trimethyl ammonium bromide (CTAB) with thiolated species, AuNPs using CTAB as a surfactant could be detoxed and stably dispersed [81, 82]. Gold-sulfur bonding was utilized by Jiang et al. [83] to create stably dispersed AuNPs with varying sizes—about 2 nm, 4 nm, and 6 nm, covered with zwitterionic ligands. Alea-Reyes et al. [84] produced AuNPs with a size of roughly 5 nm stabilized with double-pyridine salt for use in cancer treatment. Han et al. [85] combined DNA with trimethyl ammonium mixed monolayer protection cluster-adjusted AuNPs to construct a DNA-delivery device via electrostatic interactions. Kim et al. [86] synthesized triethylenetetramine-terminated ligands with a 2 nm gold core that interacted electrostatically with negatively charged siRNA.
AuNPs with a size of roughly 6 nm might be eliminated from the blood by the kidney filtering them into the bladder, as Zhou et al. [87] showed that particle size influences renal clearance efficiency. By building an AuNP illness detection platform, Loynachan et al. [88] indirectly showed that monodisperse ultrasmall AuNPs may be eliminated from the body through the kidney and liver. Therefore, the inherent qualities of monodisperse AuNPs enhance their potential for cancer diagnosis and treatment. There is an urgent need for a thorough evaluation of monodisperse AuNPs and their theragnostic applications because they have made significant strides in disease detection and therapy as a revolutionary nanomedicine.
AuNPs have potential applications in the diagnosis and treatment of cancer [89, 90]. The other side of the coin, which is the unanticipated health repercussions, must be addressed, though. Numerous studies have already been carried out to examine the physiological response, biodistribution, retention time, effectiveness, cytotoxicity, and impact of NP size on the toxicity of AuNPs. However, a lot of them seem to contradict each other. The absence of reliable data regarding the true effects of NPs could lead to problems and negatively impact human health. Although the issues raised are generally relevant to all NPs, the examples that follow are specific to AuNPs. Concern has long been raised about AuNPs’ toxicity to biological systems [91]. The toxicity of AuNPs is influenced by their size, shape, targeted ligand, surface chemistry, and composition, among other factors. It has been demonstrated that the surface charge of AuNPs affects their toxicity, with positively charged particles being more lethal than negatively or neutrally charged particles [92]. There was no toxicity found in other teams’ findings when it came to positively charged particles [93] or negatively charged AuNPs [94]. There is currently no standardized assay that can be used to detect the toxicity of all NPs, and this discrepancy stems from the unique physiochemical character of NPs.
The size and biodistribution of nanomaterials are significant factors to take into account in addition to toxicity assessment. Smaller AuNPs of about 8 nm coated with reduced glutathione were more hazardous to a human hepatic cell line than bigger particles of about 37 nm, according to Gao et al. [95]. According to Rosli et al. [96], 50 nm AuNPs were more harmful to breast cancer cells than their 13 nm and 70 nm equivalents. A variety of AuNP sizes were tested for their cytotoxicity on human leukemia cells by Connor et al. [97], who found that no size was detrimental to cellular activity. Particle form is also important. According to a study, spherical AuNPs are more absorbed by cells than rod-shaped AuNPs [98]. According to Goodman et al. [99]’s experiments, charge plays a role in toxicity as well. Positively charged particles were shown to be more toxic than negatively charged particles. Positively charged CTAB-coated AuNPs were less biocompatible with cell membranes than positively charged poly(diallyldimethyl ammonium chloride)-coated AuNPs, according to an experiment [99].
HCC is still the fourth most common cause of cancer-related deaths worldwide, and its annual global burden is rising [100–102]. The most researched and used systemic treatments in recent years have been targeted therapy and immunotherapy, which are becoming more and more crucial in the care of patients with advanced HCC [103, 104]. The primary challenges in creating customized HCC treatments are the considerable intratumoral heterogeneity of the disease and the non-negligible drug resistance of the targeted medications [105–108]. Traditional tumor models have historically hampered the development of individualized treatment for HCC by failing to capture the heterogeneity of various HCC patients or be utilized for research on targeted drug resistance in various patients. Preclinical models of liver cancer derived from patients that reflect the intricate features of tumors can now be created thanks to advancements in bioengineering techniques. This holds great promise for improving clinical outcomes and facilitating the development of personalized medicine for patients with HCC [109].
Due to the intricacy of HCC, monotherapy frequently causes significant adverse effects (AEs) that are time- or dose-dependent, which force patients to stop their treatment because they become intolerable. As a result, after an overall survival time of 14–16 months, the effectiveness of single medications like tyrosine kinase inhibitors (TKI) or immune checkpoint inhibitor (ICI) has reached a bottleneck. This implies that modifications should be made to the targeted drug development process. Thus, over the last two years, research on HCC-targeted therapy has focused on different combinations of ICI and anti-VEGF monoclonal antibodies, which has significantly increased the survival rate of patients with advanced HCC and produced a new combination for targeted therapy [110].
The transport of chemicals into cells is one of the most common uses for AuNPs. The simplicity of synthesis and functionalization, relative biocompatibility, and minimal toxicity in preliminary experiments have led to the description of AuNPs as “promising nanocarriers for therapeutics” [111–113]. However, while creating a drug delivery system, many considerations must be taken into account. It has been demonstrated that characteristics of AuNPs, such as size, charge, and surface chemistry, influence both their absorption into cells and their eventual intracellular fate (Figure 1). It’s crucial to keep an eye out for any harmful consequences of any components left in the cell after AuNPs are utilized exclusively as carriers; ideally, the NP vector should be biodegradable and have a lifespan restricted to the drug’s therapeutic window [114]. While numerous approaches have been suggested to initiate drug release at the tumor site, they can be mainly classified into three categories: photothermal or light release [115, 116], glutamate-mediated release [117], and non-covalent encapsulation of the active drug followed by membrane diffusion off-loading [118]. The others are essentially variations on one or a mix of these techniques. So far, Gholipourmalekabadi et al. [118]’s in vitro research has produced encouraging outcomes. More research is needed to determine whether these approaches are feasible for use in vivo, though. AuNPs are also being investigated as possible drug delivery vehicles for the introduction of medications into tumor cells in the context of cancer therapy [119]. Colloidal AuNPs of different sizes and shapes are reported to be absorbed by cells [120] by non-specific or specific mechanisms, such as ligand-receptor interaction (Figure 2). In order to target tumors, Huang et al. [121] have reported two techniques: the first involves conjugating AuNPs to PEG, and the second involves conjugating AuNPs with particular antibodies that bind distinctive biomarkers expressed on tumor cells. PEG prolonged AuNP retention in circulation and inhibited their aggregation. Due to the increased permeability of poorly differentiated blood vessels surrounding tumors after angiogenesis and the decreased clearance rate brought on by the absence of functional lymphatic vessels in tumors, this allowed AuNPs to accumulate preferentially in tumor cells over healthy cells [122]. It has been documented that conjugating AuNPs with methotrexate (MTX) can cause cytotoxicity in vitro and anti-tumorigenic effects in vivo [123]. Compared to tumor cells treated with free MTX, Au-MTX was found to accumulate in tumor cells more quickly and at larger concentrations. In comparison to an equivalent dosage of free MTX, this led to increased cytotoxic effects in a number of tumor cell lines. These findings are promising and point to the potential of AuNPs as drug carriers that specifically target tumor cells. They also imply that conjugating AuNPs with a chemotherapeutic agent like MTX was more effective than administering free MTX alone.
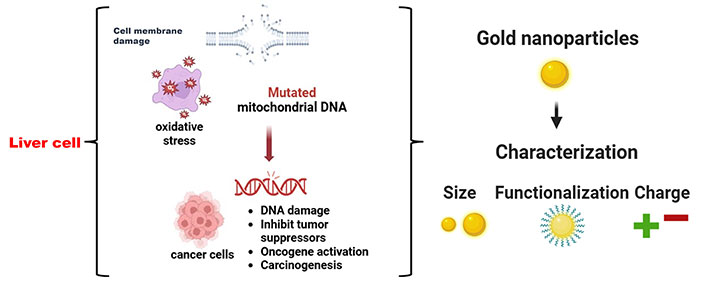
Anticancer mechanism of gold nanoparticles into cancer cells. The Figure was partially created with permission from BioRender.com
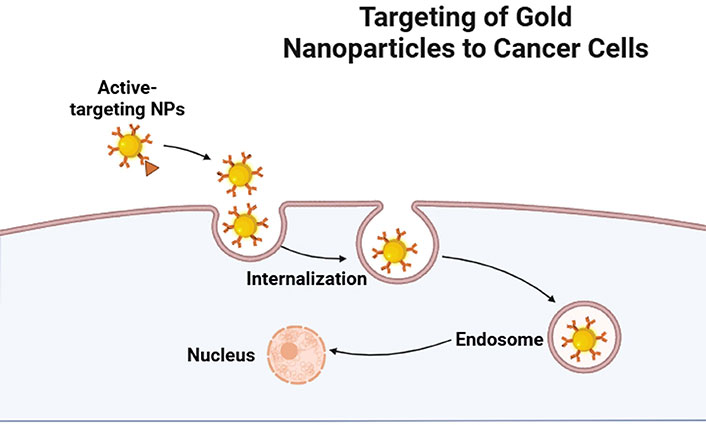
Targeting mechanism of gold nanoparticles (NPs) into cancer cells. Created with permission from BioRender.com
Comparing the biodistribution of functionalized cetuximab with the bifunctional chelating agent pisothiocyanatobenzyl-desferrioxamine moiety labeled with 89Zr (89Zr–Df–Bz–NCS–cetuximab) coupled and unconjugated to AuNPs was done utilizing quantitative PET imaging. AuNPs-plasma-polymerized allylamine (PPAA)-cetuximab-89Zr offered a high tumor-to-background ratio, according to immuno-PET study analysis, but it did not affect tumor accumulation or the effectiveness of epidermal growth factor receptor (EGFR)-targeted NPs [124]. Additionally, the surface-enhanced Raman scattering immunosensor for α-fetoprotein and telomerase measurement used gold-silica@4-mercaptobenzoic acid and gold-silica@Nile blue. Because the protein biomarkers were placed on the substrate, a limited plasmonic field was created, which increased the electromagnetic field. As a result, the molecules under investigation were exposed to a high density of “hot spots”, which increased sensitivity and the Raman signal [125]. HepG2 cells (liver cancer cells) have asialoglycoprotein receptors (ASGPRs), which makes it easier for lactobionic acid (LA)-conjugated mercaptosuccinic acid-coated AuNPs to pass through cell membranes. As a result, they could identify liver cancer cells in particular and produce a strong fluorescent signal [126]. Remarkably, doxorubicin (DOX)-loaded A-AuNC@polyacrylic acid (PAA)/mesoporous silica (mSiO2) NPs demonstrated effective tumor ablation in H-22 carrying mice without causing any systemic harm. Additionally, they have demonstrated the possibility of fluorescence imaging and dual-modal CT as contrast agents [127]. In order to enhance the surface area and biocompatibility of AuNPs and decrease the cytotoxicity of NPs, polymer films were coated. Increased liver reticuloendothelial system (RES) escape and plasma half-life were demonstrated by AuNPs-PEG [128]. In mice and rats, intravenous injections of AuNPs functionalized by gadolinium chelates were utilized as a contrast agent for both MRI and X-ray CT [129]. Compared to iodine-free AuNPs, methoxy PEG-iodine capped AuNPs significantly enhanced contrast at the heart, aorta, liver, and kidney after five days of injection in mice [130]. This was observed without any obvious harm. The X-ray attenuation property of low-generation poly(amidoamine) (PAMAM) dendrimer-stabilized AuNPs (Au DSNPs) was roughly similar to that of Omnipaque while imaging in vivo using CT. The outcomes demonstrated much greater performance than Omnipaque in CT imaging of the main organs of rats in vivo [131]. The xenoplanted tumor model and human HCC (HepG2 cells) demonstrated targeted CT imaging using the LA-modified dendrimer-entrapped AuNPs (LA-Au DENPs) both in vitro and in vivo [132]. In a model of metastatic liver cancer, the glycol chitosan (GC)-coated AuNPs (GC-AuNPs) demonstrated a tumor-targeting CT contrast agent [133]. In mice carrying CT26 tumors, the nanoprobe (MNPAPF-Au) created by co-loading AuNPs and aggregation-induced emission (AIE) red dye into 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N (DSPE)-PEG2000 micelles demonstrated a long blood circulation half-life, enhanced tumor-targeting ability, and good fluorescence and CT imaging effects [134]. When polyethylenimine-entrapped AuNPs loaded with gadolinium (Gd@Au PENPs) were injected intravenously into mice, only veins were clearly visible after small dose administration; however, with bigger dose administration, both veins and arteries were clearly seen with excellent resolution. Even at low doses of administration, MRI demonstrated veins and arteries concurrently, and at higher doses, greater resolution was observed [135]. In orthotopic liver cancer animal models, indocyanine green loaded gold nanorod@liposome core–shell NPs (Au@liposome–ICG) showed efficiency in tumor detection and surgery guiding by using photoacoustic and fluorescent dual-modality imaging probes [136]. According to a 2014 study, AuNPs made with the phytochemical 3-butoxy-2-hydroxypropyl 2-(2,4-dihydroxyphenyl) acetate from Cajanus cajan can cause apoptosis in HepG2 cells, which are used to treat liver cancer [137]. Moreover, AuNPs made by the thermophilic fungus Humicola spp. were employed to deliver drugs to hepatic malignancy, or liver cancer.
Green synthesis-produced AuNPs and the anticancer medication DOX were conjugated so that targeted drug distribution could be achieved without the use of targeting agents such as pullulan [138]. In photodynamic therapy (PDT), a photo-sensitizing (PS) chemical is administered topically or intravenously, allowed to accumulate in cancer cells, and then stimulated with a particular wavelength of light.
PDT can kill malignant cells by causing reactive oxygen species (ROS) to be released. Because PS medications are hydrophobic, one of their drawbacks in this approach is their low tissue penetration. Stated differently, it is helpful in the therapy of surface tumors [139]. AuNPs can lower this threshold by making PS medications more soluble. When they are subjected to the right visible or NIR wavelength, they can also produce heat. Furthermore, it enhances the field of light surrounding the AuNPs, which boosts PS’s excitation efficiency by LSPRs [140].
AuNPs are also a good carrier for PS medication delivery to cancer cells. However, Vankayala et al. [141]’s research shows that AuNPs’ excellent drug delivery is mostly responsible for the increased killing effect of cancer cells in PDT [142]. Additionally, singlet oxygen (1O2) can be produced by the AuNPs alone [143]. The impact of AuNPs on the effectiveness of PDT for the treatment of liver cancer has been the subject of numerous investigations to date [144–146]. Gum Arabic-conjugated AuNPs (GA-AuNPs) and laser combination studies conducted both in vitro and in vivo revealed that this technique decreases the activity of histone deacetylase in HepG2 cells as well as cell viability. The findings showed that GA-AuNPs, in combination with or instead of laser radiation, might cause cancer cells to undergo apoptosis by stimulating caspase-3 and death receptors (DRs, DR5). They can also prevent the production of preneoplastic lesions (PNLs) and their initial marker, placental glutathione S-transferase (GST-P). Moreover, laser-stimulated GA-AuNPs reduced tumor necrosis factor-α (TNF-α) levels. Thus, the GA-AuNPs and laser together triggered the extrinsic apoptotic pathway and suppressed inflammation, which can avert PNLs in the liver [147]. Au-DOX-Gel, a thermosensitive hydrogel based on Pluronic® F127, was optimized at a 22% F127 concentration to load AuNPs and develop DOX using the “cold method” for intratumoral injection. When in vitro and in vivo release characteristics were compared with the control group, they revealed continuous release of DOX and AuNPs. In human hepatocellular liver carcinoma (HepG2) and mouse melanoma (B16) cells, the combined administration of DOX and AuNPs under radiation exhibited inhibitory effects on tumor cell growth and proliferation, as well as on cell viability and the surviving percentage of the cells. When measured by Au-DOX-Gel, mouse tumor sizes were considerably smaller than those of the controls. Au-DOX-Gel can be presented as a promising technique to enhance chemotherapy because their safety was also established by skin safety testing, histological examinations of the organs, and body weight alterations [148]. Because the AuNPs are positively charged, microRNA (miR)-122 can be conjugated with them through simple electrostatic contact with folic acid-coated AuNPs (G). In HepG2 cancer cells, miR-122 releases from the AuNPs-miR-122-FA nanocomplexes and causes apoptosis [149]. According to Xue et al. [150], AuNP-miR-375 can effectively transfer miR-375 into HCC cells, suggesting that it may be utilized for HCC therapy.
PDT, a well-known non-invasive cancer treatment, has been used extensively to treat intraperitoneal tumors, cutaneous malignancies, and premalignant and malignant disorders of the head and neck with significantly lower morbidity and deformity [151–153]. When exposed to light with a wavelength that matches the photosensitizer’s absorption spectrum, the administered photosensitizer combines with nearby substrates or molecular oxygen to create ROS, which cause in situ, targeted damage to tumor tissues. Rose Bengal (RB), a well-known anionic photosensitizer among the many agents employed in PDT, with a 1O2 quantum yield of over 76% when exposed to light at 532 nm [154]. However, because of its limited intracellular absorption capabilities as a hydrophilic photosensitizer, RB is not suitable for treating solid malignancies [155]. The use of NPs as a carrier to conjugate with RB to increase the absorption efficiency by cancer cells has been demonstrated in recent research [156]. Additionally, by incorporating the DNA polymerases of cancer cells, RB, an anionic water-soluble xanthene dye, has been shown to have selectivity to oral cancer cells [157].
Gold nanoshells functionalized with a short peptide have been used in the photothermal treatment of hepatocarcinoma, according to research by Liu et al. [158]. With minimal cytotoxic action, the functionalized gold nanoshells have demonstrated good targeting performance in liver tumor cells BEL-7404 and BEL-7402; nevertheless, they are ineffective against HL-7702, a normal, healthy liver cell. Additionally, their fluorescence photos have demonstrated that, following treatment with a NIR laser irradiation, the gold nanoshells may cause the liver cancer cells to die during in vitro trials. A semiconducting polymer was employed by Sun et al. [159] for orthotopic liver cancer treatment under laser irradiation. They have demonstrated that, under comparable circumstances, a 1,064 nm laser inhibits the growth of orthotopic liver cancer cells more effectively than an 808 nm laser [160, 161]. AuNPs are suitable for photothermal cancer treatment since they produce heat when exposed to NIR laser light [162, 163]. For imaging and photothermal therapy, Xia et al. [164] produced AuNPs with a size of roughly 12 nm using folic acid and the reduction of a bovine serum albumin conjugation. In addition, several techniques, including electrostatic adsorption, are employed to modify the surface of AuNPs via the thiol gold reaction. Additionally, gold nanorods (AuNRs) covered with mSiO2, also known as Janus NPs, with a core-shell type demonstrated efficient entry into HepG2 cells for both photothermal effect and cellular imaging [165].
Most of the research that has been included in this review thus far has emphasized how generally safe AuNPs are. Nonetheless, a number of additional investigations have sparked worries about these NPs’ potential toxicity. It has been established that the physicochemical characteristics of AuNPs, including their size, charge, shape, and surface chemistry, affect their toxicity. According to an in vitro investigation, AuNPs shielded by Ph2PC6H4SO3Na and P(C6H4SO3Na)3 ligands exhibited toxicity toward various cell lines that were both size-dependent and cell type-independent [166]. Up to 60 times more cytotoxicity was demonstrated by 1.4 nm AuNPs than by 15 nm AuNPs. Subsequent research by the same working group revealed that 1.4 nm triphenylphosphine monosulfonate-coated AuNPs induce the production of ROS, up-regulation of genes linked to stress and inflammation, a shift in mitochondrial permeability, and ultimately the death of HeLa human cervix carcinoma cells [167]. According to another study, neither 13 nm nor 45 nm AuNPs can enter the human dermal fibroblasts’ nucleus or mitochondria because they remain in cytoplasmic vacuoles [168]. They also reported that 45 nm AuNPs also caused more cell damage because of their distinct uptake mechanism, which led to an increased release in the cytoplasm. An in vivo investigation revealed that the toxicity of AuNPs following intraperitoneal and oral administration is greater than that following tail vein administration [169]. Another in vivo study using Drosophila melanogaster demonstrated that 15 nm naked AuNPs had a mutagenic effect [170]. These investigations demonstrated that the intrinsic properties of AuNPs, specifically their size, shape, charge, and surface ligand, are the primary cause of their toxicity [171]. Therefore, more research is needed to determine the best combination of AuNP characteristics for targeted drug delivery in order to address toxicity issues.
Metallic NPs have demonstrated a valuable and prevalent role in the last 20 years in the treatment of cancer by offering better drug delivery and targeting. Furthermore, they provide outstanding control over energy deposition in tumors through their functionalization with targeting ligands. Gold has been used in medicine for centuries due to its bacteriostatic, anti-oxidative, and anti-corrosive qualities. Furthermore, due to its photothermal and photoacoustic characteristics, as well as its nanoscale synthesis and functionalization with a variety of drugs and targeting molecules, AuNPs have gained recognition as an excellent multifunctional material for cancer therapeutics. PET, CT, and MRI were the most widely used AuNPs-based liver tumor imaging techniques. Because of their high ability to load genes and drugs onto their surface, AuNPs delivery systems have demonstrated encouraging results in the treatment of liver cancer.
AuNPs: gold nanoparticles
CT: computed tomography
CTAB: cetyl trimethyl ammonium bromide
DOX: doxorubicin
GA-AuNPs: Gum Arabic-conjugated gold nanoparticles
HCC: hepatocellular carcinoma
LA: lactobionic acid
LSPR: localized surface plasmon resonance
miR: microRNA
MRI: magnetic resonance imaging
MTX: methotrexate
NIR: near-infrared
NPs: nanoparticles
PDT: photodynamic therapy
PEG: polyethylene glycol
PS: photo-sensitizing
RB: Rose Bengal
ROS: reactive oxygen species
SPR: surface plasmon resonance
The authors have utilized the highly esteemed software, Biorender, to create the figures for this manuscript. BioRender.com (2024). Retrieved from https://www.biorender.com/.
SS: Data curation, Writing—original draft, Supervision. MT, SD, and AB: Writing—review & editing.
The authors declare that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2958
Download: 32
Times Cited: 0
