Abstract
Systemic hypertension, a significant global health issue and a leading risk factor for cardiovascular mortality affects half of the adult population, with increasing prevalence notably in low- and middle-income countries. Despite advancements in diagnosis and treatment, only one in four individuals with hypertension achieve satisfactory control over their condition. Medication adherence, critical for effective hypertension management, is complex and multifaceted. Non-adherence, encompassing late or non-initiation, sub-optimal implementation, and early discontinuation of treatment, is prevalent worldwide, with reported rates of anti-hypertensive medication non-adherence ranging from 30% to 40%. Adherence is influenced by various factors including drug regimen complexity, patient education, and socioeconomic status. Poor adherence is linked to increased cardiovascular risks and is compounded by clinical inertia among physicians. Addressing barriers to adherence and implementing evidence-based interventions can significantly reduce the global burden of hypertension and its associated complications. This review highlighted the critical need for improved adherence strategies to enhance hypertension management. It focused on novel tools such as mobile health interventions and regimen-simplification through single-pill combinations, which can improve treatment persistence and blood pressure control.
Keywords
Adherence, non-adherence, arterial, hypertension, narrative reviewIntroduction
Hypertension is a major health concern, as well as the leading preventable risk factor for mortality and disability worldwide [1]. It is defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic BP (DBP) ≥ 90 mmHg [2, 3]. Despite the fixed diagnostic threshold, it is well known that values of SBP above 115 mmHg and DBP above 75 mmHg correlate with a continuous increase in cardiovascular (CV) or renal events [2].
In 2023, the World Health Organization (WHO) published its first “Global report on hypertension”. According to the report, hypertension is estimated to affect 33% of adults aged 30–79 worldwide. Furthermore, the number of adults with hypertension doubled from 650 million in 1990 to 1.3 billion in 2019. Despite hypertension being such a high prevalence condition, only 54% of affected adults are diagnosed, 42% receive treatment, and a mere 21% have their BP controlled. In addition, the report points out that approximately three-quarters of individuals with hypertension live in low- and middle-income countries, with the prevalence of this condition higher in such countries than in high-income ones [4].
A systematic analysis of population-based studies from 90 countries, which included more than 968,000 adults, showed that the prevalence of hypertension decreased by 2.6% in high-income countries while it increased by 7.7% in low- and middle-income countries from 2000 to 2010. This rapid increase in the burden of hypertension is most likely due to both increasing prevalence and substantial population growth in low- and middle-income countries [5].
The 2023 European Society of Hypertension (ESH) guidelines for the management of arterial hypertension identified that poor adherence to treatment and physician clinical inertia (failure to initiate or intensify therapy appropriately when patients have uncontrolled parameters) are among the main causes of poor BP control [2, 6–8].
In real-life settings, the optimization of anti-hypertensive treatment is often affected by medical inertia. In fact, the initial administration of two anti-hypertensive drugs, and up-titration to three or more drugs when necessary, is significantly less frequent than that shown to be necessary in clinical trials and guidelines [6].
A cohort study by Ali et al. [9] showed that among patients with uncontrolled BP in primary care, therapeutic inertia was 87%, and it was significantly associated with older age, diabetes, and BP values close to the target. The insufficient implementation of anti-hypertensive therapy appears to be due to the fact that general physicians often do not consider office BP measurement representatives, they tend to wait for another BP measurement, and they prefer trying to achieve BP control with optimization of lifestyle measurements.
On the other hand, poor adherence to anti-hypertensive medication has been associated with an increased risk of CV complications, CV, and cerebrovascular mortality in several studies [10–12]. Medication adherence also plays a significant role in the quality of life of hypertensive patients. A recent study showed an inverse correlation between quality of life and adherence, suggesting that greater adherence implies better quality of life [13]. Furthermore, non-adherence has also been linked to potentially inappropriate increases in treatment intensity [14].
Understanding the factors associated with medication non-adherence and how to address it, is essential to reduce mortality and adverse events in hypertensive patients, as well as to reduce unnecessary costs derived from changes in treatment regimens and from further medical complications.
Definition of adherence to medications
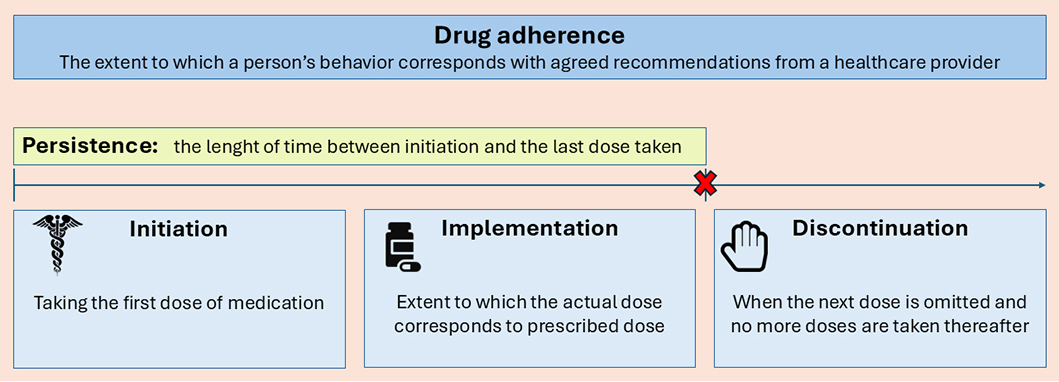
In the past years, many terms have been used to indicate adherence to medications, such as adherence, compliance, persistence, and concordance. However, the meanings and the difference between these terms have not always been clear and uniform. In 2003, the WHO defined adherence as “the extent to which a person’s behavior—taking medication, following a diet, and executing lifestyle changes, corresponds with agreed recommendations from a health care provider” [15]. In 2009, a European consensus meeting took place with the aim of defining a new taxonomy for adherence to medications. Adherence was divided into three components: ‘initiation’—when the patient takes the first dose of a prescribed medication; ‘implementation’ of the dosing regimen—the extent to which a patient’s actual dosing corresponds to the prescribed dosing regimen; ‘discontinuation’—the end of the therapy when the next dose to be taken is omitted and no more doses are taken thereafter. ‘Persistence’ is defined as the length of time between initiation and the last dose, which immediately precedes discontinuation. Because of negative connotations, ‘compliance’ is not the most appropriate term to use when referring to patients following therapeutic recommendations [16]. Non-adherence to medications can be the result of late or non-initiation, a sub-optimal implementation of the dosing regimen, early discontinuation, or a combination of any of the above (Figure 1).
Non-adherence to anti-hypertensive treatments is a multifactorial, complex phenomenon. The fact that hypertension is generally an asymptomatic condition, is likely to contribute to the risk of non-adherence when compared with other CV conditions known to produce symptoms when treatments are not followed, such as chronic heart failure, which is associated with much better drug adherence [17].
Non-persistence is one of the most common causes of poor adherence in hypertension, particularly among newly treated patients. Non-persistence and low adherence result from both intentional (i.e., not taking medication because of the fear of side effects) and unintentional reasons (i.e., forgetfulness) [18], being unintentional the more common reasons [19].
Sociodemographic and economic factors, such as age, and ethnicity, may impact adherence [20]. Several studies have shown that young patients are less likely to be adherent to anti-hypertensive therapy [21, 22]. Low educational level, low income, poor health status, non-white race, depressive symptoms, and history of chronic heart disease are all factors that may reduce anti-hypertensive drug adherence [22, 23]. Finally, hospitalization events may positively affect adherence, because of the increased motivation and higher expected quality of the hospital care compared to the outpatient care [22].
A 2022 meta-analysis by Lee et al. [24] reported that the global prevalence of anti-hypertensive medication non-adherence ranged from 27% to 40%. Furthermore, anti-hypertensive medication non-adherence was more prevalent in low-to-middle-income countries and non-Western countries. In clinical studies, 4% to 5% of patients never start their treatment, despite accepting to be enrolled in the study. However, non-initiation appears much more frequent in clinical practice: up to 24% in patients treated for hypertension but also in those treated for diabetes mellitus or dyslipidemia. However, this phenomenon may vary considerably depending on the countries and the access to medications [19, 25].
It is not possible to define drug adherence quantitatively with a given threshold. A cut-off of 80% has been frequently used to define good adherence, even though there is poor evidence that this cut-off is relevant [26].
Many factors can influence the clinical consequences of missed doses on BP control, such as the duration of action of the prescribed drugs, and the grade of hypertension [e.g., mild hypertension or severe resistant hypertension (RH)] [26].
The extent of non-adherence to anti-hypertensive drugs shows a strong correlation with the degree of BP elevation, in terms of both office BP measurements and 24-hour ambulatory BP monitoring (ABPM) [27].
Assessment of drug adherence
Non-adherence to BP lowering therapy is common, particularly in patients with suboptimal BP control. The 2023 ESH guidelines for the management of arterial hypertension [2] recommend the screening for non-adherence in all patients with apparently RH (I-B), and in patients who are on combination treatment (at least 2 drugs) and have an inadequate BP response (II-C). In addition, they recommend checking adherence prior to screening for secondary hypertension (I-C). Similar indications are given by the 2024 ESC guidelines for the management of elevated BP and hypertension, which recommend evaluating adherence in the clinical workup of patients with apparent RH (IIa-B) [3].
Hypertension is defined as resistant to treatment when office BP is ≥ 140/90 mmHg despite appropriate lifestyle measures and treatment with an adequate dose of three or more drugs, including a thiazide or thiazide-like diuretic, a renin-angiotensin system blocker and a calcium channel antagonist. However, to define ‘true RH’, evidence of adherence and exclusion of secondary causes of hypertension are required, otherwise, the condition is defined as ‘pseudo-RH’. After ruling out poor adherence and secondary causes, the estimated prevalence of true RH is about 5% of the total hypertensive population [2]. Thus, it is important to assess drug adherence in patients with uncontrolled BP, to decide whether to focus the efforts on improving adherence to the current prescribed regimen or on intensifying the treatment regimen [28].
Several methods have been used to evaluate adherence (Figure 2), each of which has strengths and weaknesses, but as of today none of them can be considered as gold standard. The ideal method for assessing anti-hypertensive drug adherence should be reliable, easy to apply, and relatively inexpensive, providing information on the dosing history and verifying drug intake. However, there is no method that meets all these criteria. In addition, the assessment of adherence is often biased by patients’ awareness that their behavior is being monitored (the so-called Hawthorne effect); thus, the result of drug adherence evaluation may be affected, particularly in a research setting [20].
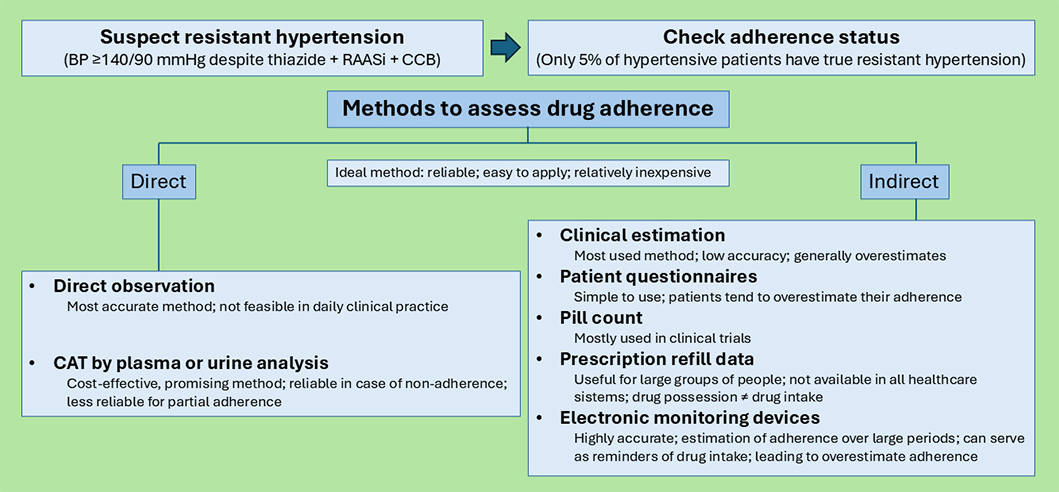
Methods to assess drug adherence. BP: blood pressure; RAASi: renin-angiotensin-aldosterone system inhibitor; CCB: calcium channel blocker; CAT: chemical adherence testing
Adherence assessment methods can be divided into indirect and direct. Indirect methods include clinician estimation, patient questionnaires, pill counting, prescription refill data, and electronic monitoring (EM). Direct methods include direct observation of therapy and chemical adherence testing (CAT) by plasma or urine analysis [20]. Traditionally, the most used method in daily practice is the clinical estimation of drug adherence by physicians, an approach known to be unreliable. Physicians’ perception of whether a patient is adherent to the anti-hypertensive medications or not has been shown to be wrong in up to 50% of cases, generally overestimating adherence [28, 29]. Another approach to measuring drug adherence is self-reporting, using detailed adherence questionnaires [30]. Numerous questionnaires have been validated to measure adherence to anti-hypertensive drugs [31], but none of them can be considered a gold standard [31]. Among these, the Morisky Medication Adherence Scale (MMAS) has been widely utilized; however, its validity has been questioned following the retraction of key studies underpinning its use [32]. These questionnaires are relatively simple to use and in general more reliable than unstructured self-reported adherence. However, assessing adherence based on patients’ self-reported information by adherence questionnaires is considered inaccurate when compared to direct measures, as patients tend to overestimate their adherence [33–35]. Pill count is an indirect method, often used in clinical trials. It is relatively simple to use, providing an overview of the number of pills taken by the patient by counting the remaining pills in the boxes. However, studies have demonstrated its tendency to overestimate adherence [19, 36].
Digital sensors are a promising emerging technology based on the use of pills equipped with biodegradable sensors, to capture timing and frequency of pill swallowing. These sensors provide an accurate estimation of the level of adherence. They have been applied to the treatment of psychiatric disorders, but they are not yet available for anti-hypertensive drug monitoring [33, 37].
Electronic drug monitoring devices were developed to capture the time and frequency of medication use by tracking medication bottle cap opening [38]. The use of EM is considered a highly accurate and objective method to assess drug adherence, as it can provide a dosing history over months or years. However, these devices and the patients’ awareness of being observed may serve as reminders themselves, leading to an overestimation of the true adherence. Furthermore, EM devices are unable to certify the ingestion of the correct drug or the correct dose: since they only detect the bottle opening, patients may not actually take the medications after opening the bottles, and they also may remove multiple doses at once for later administration. In addition, as they are able to monitor only one medication per bottle, it may be challenging to implement EM devices for patients on multiple medications [33, 39]. Their use is still mostly limited to research settings [20].
Pharmacy refill data are an inexpensive and easy-to-obtain indicator of adherence. If linked to prescribing data, they can be used to identify non-initiation [25]. In this context, continuous multiple-interval gap (CMG) is a measure that can be used to determine what percentage of days over the past year the patient did not possess the prescribed BP medications [28]. Studying these data may be the easiest way to obtain information about the behavior of large groups of patients. However, not all health systems have access to such data. Furthermore, the information provided is whether the patient is in possession of the medication or not, and this does not ultimately translate to drug intake by the patient.
Direct observation of medication intake is the most accurate method of determining adherence but is generally not feasible in clinical practice. It consists of supervising directly if the patient swallows the pills [40, 41].
Another direct method of assessment of drug adherence is CAT by serum or urine analysis. An increasing number of studies show that CAT is reliable for detecting medication non-adherence in patients who seem to have RH [27, 34, 42, 43]. A study on 208 hypertensive patients attending a specialist hypertension centre in the UK evaluated the prevalence of anti-hypertensive treatment non-adherence by a simple urine-based assay (qualitative high-performance liquid chromatography-tandem mass spectrometry, HPLC-MS/MS). The study showed that one in four patients were partially or totally non-adherent to pharmacological BP-lowering therapy. Interestingly, the same study showed for the first time alarmingly high levels of complete biochemical non-adherence to treatment among patients referred for renal denervation for RH [27]. Both urine and serum CAT are widely available for clinical use and are covered by most health insurance plans. Brinker et al. [34] found the incremental cost of CAT to be under $5.00 per mmHg-reduction in SBP, far less than the cost associated with device therapies such as renal sympathetic denervation. Non-detectable drug levels are a strong marker of non-adherence, but drug levels could be inconclusive in cases of partial adherence. Unfortunately, current methods of biochemical assessment do not provide an assessment of adherence to the therapeutic level of anti-hypertensive medications [33]. The use of CAT as a direct method for evaluating adherence is producing promising results and is expected to become standard practice in the context of RH [44].
Methods to improve drug adherence
Several methods can be used to improve drug adherence (Figure 3). Firstly, physicians should provide extensive therapeutic education to hypertensive patients, especially to those at higher risk of drug non-adherence. Providing an explanation about drugs’ purpose and the need for continued drug adherence and addressing specific patient concerns may be effective for improving drug adherence. It is important to enhance patients’ empowerment and to promote self-management skills that can be useful in improving drug adherence [20].
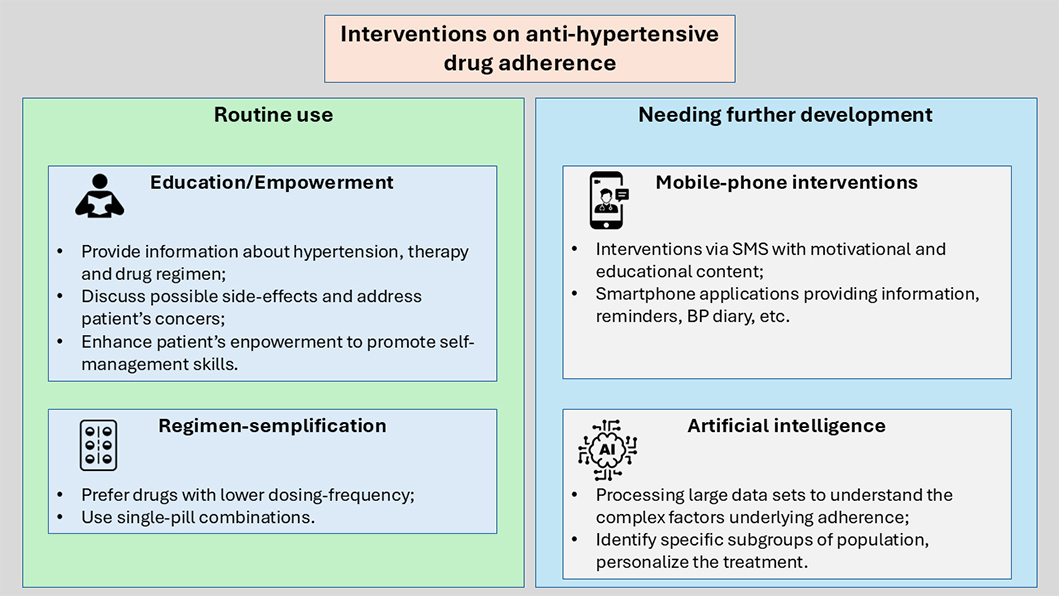
Interventions on anti-hypertensive drug adherence. SMS: short text messages; BP: blood pressure
Also, regimen-simplification is an important tool to improve adherence and BP control. Dosing frequency has been shown to have a negative influence on drug adherence, being the once-daily regimens associated with significantly higher adherence than twice-daily, three-times-daily, and four-times-daily regimens [45, 46]. Moreover, hypertensive patients receiving single-pill combinations (SPCs) are significantly less likely to discontinue therapy than patients receiving free-equivalent combinations (FECs), resulting in significantly better BP control, reduced CV event rates, and all-cause mortality [47–49].
The latest European Society of Cardiology and ESH guidelines recommend the use of SPCs to improve adherence and persistence [2, 3]. The role of mobile-phone interventions in improving anti-hypertensive drug adherence has been evaluated in several studies, using various forms of intervention. Short text messages (SMS) that provide motivation and education about hypertension treatment and healthy diet, along with medication schedules were assessed in several studies [50, 51]. Other groups utilized smartphone applications with educational content about hypertension, drug intake reminders, routine visits reminders, BP diary, and notes by the physicians [52]; in some studies, smartphone applications linked to external monitoring devices were used, sending periodic reminders about drug intake and BP monitoring [53, 54]. These studies showed a tendency of increased adherence to anti-hypertensive therapy and BP monitoring as a result of mobile-phone interventions, although there are differences in the modality and frequency of reminders between studies. However, mobile-phone intervention should be developed further before being applied in daily practice [55].
A meta-analysis of 16 RCTs (randomized controlled trials) assessed the effect of mobile telephone text message interventions to promote medication adherence in adults with chronic diseases, including CV disease. According to this study, mobile-phone text messaging approximately doubles the odds of medication adherence [56].
A growing number of studies have suggested the use of artificial intelligence (AI) and machine learning (ML) both to evaluate and improve drug adherence [57, 58]. ML methods can also be used to examine the influence of drug adherence on clinical outcomes. In a study involving more than 30,000 patients with type 2 diabetes mellitus, ML was used to examine the association between adherence to oral hypoglycaemic drugs and hospitalization events [58]. ML has also been used to develop a prediction model to identify the key factors associated with medication adherence and treatment success of hypertension in the New York City population. Age, race, neighborhood poverty, history of diabetes mellitus, household income, and health insurance coverage resulted in the most reliable factors to predict medication adherence. Among these, age was the most important predictor [59].
Processing large and complex data sets in healthcare with AI technologies can help us advance our knowledge of the complex factors underlying medication adherence and, as a result, increase our comprehension of how to improve it [60, 61]. This can be achieved especially by ML and big data analytics, allowing to identify and address specific subgroups, in order to personalize the treatment and promote an approach based on precision medicine [62, 63].
Guthrie et al. [62] studied the effect of interventions delivered through a smartphone application that provides tools to support the patient in behavioral changes and self-monitoring to reduce BP and improve cardio-metabolic profile. The app uses AI to provide feedback to the patient to enhance adherence to behavioral therapy. The interventions resulted effective in reducing BP values, with a rapid effect observed starting from the first six weeks, and a greater effect observed in the patients with higher BP at baseline.
Another comprehensive method of digital intervention was developed by Silva et al. [64]. It consists of an application connected to a system of devices including smartphones, smart TVs, smart watches, a BP meter, and other gadgets, all connected to a server with an internet connection. The application records data from these devices, the BP measurements are added to the system and, based on the medical prescription, feedbacks are delivered through a smart medicine cabinet and by dialog screens in smartphones or smart TVs (e.g., alerts at a certain time to take medication or to measure BP). This system uses ML algorithms to analyze the medication intake and BP values to automatically determine the level of adherence of the patient. Although not ready yet for clinical application, the system shows promise for future broad application particularly for unaccompanied and elderly patients.
Conclusions
Systemic hypertension remains a pressing global health issue, exacerbated by significant challenges in medication adherence. Despite the availability of effective treatments, achieving optimal BP control is still elusive for many patients. This review underscored the complexity of adherence, which is influenced by factors such as medication regimen complexity, patient education, and socioeconomic status. Non-adherence, whether due to late initiation, sub-optimal implementation, or early discontinuation of treatment, poses a substantial barrier to effective hypertension management.
The prevalence of hypertension is notably higher in low- and middle-income countries, where the burden of disease is increasing due to both rising prevalence and population growth. Addressing non-adherence in these settings is particularly critical, as the impact on CV morbidity and mortality is profound. Clinical inertia, characterized by the failure to intensify treatment appropriately, further compounds the issue, highlighting the need for both patient and physician-focused interventions.
Recent advances in adherence assessment and intervention strategies offer promising avenues to improve outcomes. Mobile health interventions, such as text message reminders, have demonstrated significant potential in enhancing medication adherence. Similarly, simplifying treatment regimens through SPCs has been shown to improve persistence and BP control. These approaches are supported by current guidelines and represent practical steps that can be widely implemented.
AI and ML are emerging as powerful tools to both assess and enhance medication adherence. These technologies can process large datasets to identify patterns and predictors of non-adherence, allowing for more targeted and effective interventions. By leveraging AI, healthcare providers can develop personalized adherence strategies that address the unique barriers faced by each patient.
Ultimately, improving medication adherence requires a multifaceted approach that addresses the diverse factors contributing to non-adherence. Patient education and empowerment are central to this effort, ensuring that patients understand the importance of adherence and are equipped to manage their treatment effectively. Healthcare systems must also prioritize adherence monitoring and support, integrating these practices into routine care.
The global burden of hypertension and its associated complications can be significantly reduced through concerted efforts to improve medication adherence. By embracing innovative technologies, simplifying treatment regimens, and fostering a patient-centred approach, we can enhance hypertension management and ultimately improve patient outcomes. Continued research and investment in adherence strategies are essential to achieving these goals and addressing the critical challenge of hypertension on a global scale.
Abbreviations
| AI: | artificial intelligence |
| CAT: | chemical adherence testing |
| CV: | cardiovascular |
| DBP: | diastolic blood pressure |
| EM: | electronic monitoring |
| ESH: | European Society of Hypertension |
| ML: | machine learning |
| RH: | resistant hypertension |
| SBP: | systolic blood pressure |
| SPCs: | single-pill combinations |
| WHO: | World Health Organization |
Declarations
Author contributions
CT: Conceptualization, Writing—original draft. MT: Conceptualization, Writing—review & editing. Both authors approved the final version of the manuscript.
Conflicts of interest
Both authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.