Abstract
Aim:
Non-adherence is an important cause of uncontrolled hypertension. We investigated the prevalence of non-adherence to antihypertensive medications by serum drug concentration measurements in a cohort of Norwegian patients from the general population treated for hypertension. We also identified characteristics associated with non-adherence.
Methods:
Patients with hypertension using ≥ 2 antihypertensive agents were invited to participate in this national study performed in a semi-blinded fashion. Exclusion criteria were dementia, substance or alcohol abuse, pregnancy, terminal illnesses, poor Norwegian language skills, or severe kidney failure [glomerular filtration rate (GFR) < 30 mL/min/1.73 m2]. All patients had their antihypertensive drugs in serum analyzed by ultra-high-pressure liquid chromatography-tandem mass spectrometry to identify their adherence to the 23 most used antihypertensive agents. Additionally, they underwent a thorough standardized interview, office blood pressure (BP), and 24-hour ambulatory BP measurement.
Results:
n = 1,151 patients were investigated for BP control and drug adherence. Among these, n = 79 (6.9%) patients were identified as non-adherent, and n = 1,072 (93.1%) patients were identified as adherent by pharmacologists who reviewed the drug concentrations in blood in relation to self-reported prescribed medications. We found the non-adherent patients to be younger (56.9 vs. 63.7 years, p < 0.001), with higher systolic and diastolic office BP (150.4/91.4 vs. 143.2/82.0 mmHg, p < 0.01) and less likely to be of European ethnicity (82.9% vs. 95.8%, p < 0.001). Factors associated with non-adherence in logistic regression analysis were age, number of antihypertensive pills, non-European ethnicity, and inversely the use of angiotensin receptor blockers.
Conclusions:
We found fewer non-adherent patients than expected in patients on ≥ 2 antihypertensive drugs compared to previous investigations of patients on ≥ 3 antihypertensive drugs. We believe that selection at inclusion or possibly a higher confidence in physicians’ authority may be of importance. Adherence was significantly and negatively related to younger age, non-European ethnicity, and increasing number of prescribed antihypertensive pills, and positively to the type of prescribed medication, especially angiotensin receptor blocker (www.ClinicalTrials.gov identifier: NCT03209154).
Keywords
Adherence, antihypertensive medication, blood pressure, hypertensionIntroduction
According to the World Health Organization, ensuring a normal blood pressure (BP) is a number one priority in preventing cardiovascular morbidity and mortality in hypertensive patients [1, 2]. Studies have shown that most hypertensive patients do not reach their BP target, giving them an increased risk of cardiovascular disease. Doctors’ inertia is a large contributing factor to such uncontrolled BP [3], but also poor drug adherence plays a major role. Even though cheap and effective antihypertensive medication is available, about one in three, to one in four, patients on average are non-adherent according to the European Society of Hypertension guidelines [2], making it difficult to reach the individual’s BP goal.
There are numerous different reasons why patients do not take their medications as prescribed, i.e., side-effects or fear of side-effects, communication issues in the doctor-patient relationship, and surprisingly, a too high number of prescribed pills [4]. The incidence of non-adherence differs widely in various studies [5–8]. Several methods have been used to estimate the number of non-adherent patients: pill counting, electronic pill boxes, counting prescriptions, or self-reporting [9–11]. The method used for assessing adherence will impact the level of adherence found in the study [12]. In later years blood and urine samples have been used to measure drug concentrations directly and thereby assess adherence, providing a more valid and objective, but also a more costly method to investigate adherence [13] compared to subjective and cheaper methods like pill-counting and self-reporting [6].
Adherence has mostly been investigated in special populations of hypertensive patients on 3–5 or more antihypertensive drugs [12, 13]. Therefore, in the present study, we aimed to investigate the prevalence of non-adherence in more regular hypertensive patients on ≥ 2 drugs and recruited from the general population. To include such a population, we performed a national study of hypertensive patients and we also identified characteristics and variables associated with non-adherence. To minimize potential bias, we performed study procedures in a semi-blinded or even blinded fashion; investigators were unaware of the adherence status of the participating patients, and BPs were taken by technicians with otherwise minimal or no patient contact.
Materials and methods
Material
We performed a Norwegian cross-sectional multicenter cohort study with public funding. Investigators from all medical universities in the country participated. This study aimed to describe a Norwegian population-based cohort on standard antihypertensive treatment, and to investigate drug adherence in patients with uncontrolled hypertension by measuring serum concentrations of commonly used antihypertensive drugs in the country.
Patients were included at four clinical centers: namely at the university hospitals in Oslo, Trondheim, Bergen, and Tromsø. Most patients were referred by general practitioners (GPs), but some were self-referred (responding to advertisements in media) or referred by nephrologists or cardiologists in hospital-based or private outpatient clinics. Participation was free of charge.
Inclusion and exclusion criteria
To meet the inclusion criteria, patients had to be ≥ 18 years of age, being prescribed ≥ 2 antihypertensive agents, and with a stable drug regimen during the last four weeks. Exclusion criteria comprised pregnancy, terminal illness, drug or alcohol abuse, dementia, poor Norwegian language skills, or chronic kidney disease with estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2, or urine albumin/creatinine ratio > 300 mg/mmol. All patients gave written informed consent, and the study protocol was approved by the Regional Ethical Committee of the UiT Arctic University in Norway, Tromsø, Norway (approval no. 2017/804/REK Nord). This Ethical Committee had the authority of making approval for all participating centers including subsequent changes in investigator staff, study documents, and announcements needed to recruit patients to the study.
Study design
A comprehensive description of the study design has been published previously in a half-way report [6]. In short, patients underwent a structured physician-patient interview after signing the informed consent. This interview provided information about demographic data, background, socioeconomic status (income, education, and occupation), medical history, family history, and previous treatment. All information on antihypertensive and concomitant treatment was self-reported by the patients. The total number of prescribed daily antihypertensive and concomitant pills was calculated by summing all prescribed pills per week divided by seven. After completed interview, the patients had blood samples and BPs taken. A noteworthy point in the design is that the patients were uninformed about the intention to analyze blood samples for serum drug concentrations and thereby investigate for nonadherence. This procedure was approved by the Ethical Committee and done to minimize so-called white-coat adherence.
BP measurements
All patients had office BP and ambulatory BP measurement (ABPM) taken with a validated device by a standardized procedure as described by the European Society of Hypertension guidelines [2]. Validated devices used in the study were Microlife WatchBP O3 (Microlife Health Management Ltd., Cambridge, UK), SunTech Oscar 2 (SunTech Medical, Morrisville, NC, USA), or Omron HEM-907 (Omron Corporation, Kyoto, Japan). Seated office BP was taken after 5 min of rest. Three measures were performed with 1 min rest between measurements, and we used the average of the last two measurements. After office measurements, the same device was used for subsequent 24 hours ABPM. Patients with a mean daytime systolic BP ≥ 135 mmHg were defined as having uncontrolled hypertension and had further follow-up visits in the main study.
Blood sample and urine testing
All patients had their blood samples drawn and analyzed for a panel of biomarkers. In addition, a 5 mL Vacutainer tube without additives was collected for pharmacological analyses. The further preparation method has been described previously in detail [14]. Patients also brought morning urine samples for examination.
Pharmacological assessment
The pharmacological serum samples from all four centers were analyzed at the Department of Pharmacology at Oslo University Hospital. By use of ultra-high-pressure liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) [Agilent 1290 Infinity UHPLC combined with Agilent 6490 MS/MS (Matrix, Oslo, Norway)] the 23 most frequently prescribed antihypertensive agents in Norway were analyzed (Table 1). Experienced pharmacologists considered the patients’ drug concentrations and indicated adherence or non-adherence when related to their self-reported prescribed antihypertensive medications, dose of medications, and time of intake. Patients were defined as adherent if all prescribed antihypertensive agents were above a predefined cut-off value [6]. If one or more agents were below the cut-off value, the patient was designated as non-adherent.
Antihypertensive agents or their metabolites investigated by serum measurements in the present study
| Drug classes | Antihypertensive agents/metabolites |
|---|---|
| Selective alpha-adrenoreceptor blockers | Doxazosin |
| Beta-blockers | Atenolol |
| Bisoprolol | |
| Carvedilol | |
| Labetolol | |
| Metoprolol | |
| Calcium channel blockers | Amlodipine |
| Diltiazem | |
| Lercanidipine | |
| Nifedipine | |
| Verapamil | |
| Angiotensin converting enzyme inhibitors | Enalaprilat |
| Lisinopril | |
| Ramiprilat | |
| Angiotensin receptor blockers | Candesartan |
| Irbesartan | |
| Losartan carboxylic acid | |
| Telmisartan | |
| Valsartan | |
| Thiazide diuretics | Bendroflumethiazide |
| Hydrochlorothiazide | |
| Aldosterone antagonists | Canrenone |
| Eplerenone |
Statistical analyses
All analyses were performed in SPSS 29.0 (Chicago, IL, USA). Continuous variables are shown as mean [standard deviation (SD)] and categorical variables as absolute numbers with percentages. Q-Q plots and Shapiro-Wilk test for normality were performed to verify normal distribution of samples. Fisher’s exact test or chi-square was used to compare differences between categorical variables, and students’ T-test for comparison of continuous variables. Two-sided p-value < 0.05 denoted statistical significance.
We used logistic regression analyses to identify variables associated with non-adherence. Variables with a p < 0.10 when testing for differences between groups, were included in univariate logistic regression analyses with non-adherence being the dependent variable. Significant univariate factors were included in the multivariate analyses by stepwise regression deciding the final model. We performed backward regression analysis and tested for interactions between the different covariates.
Results
Characteristics
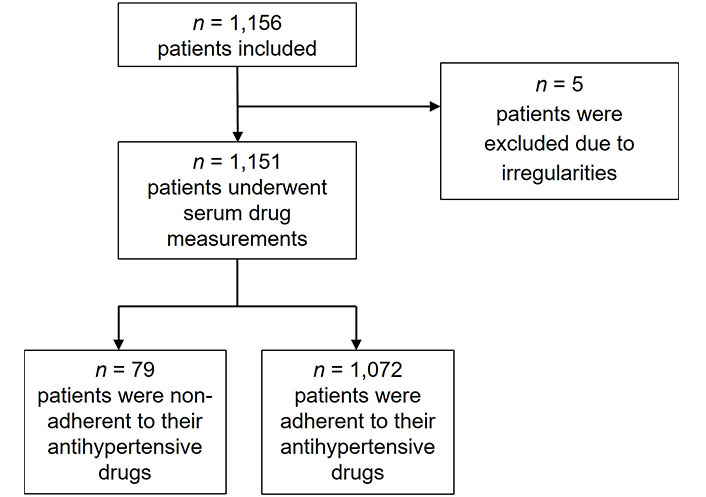
Totally, n = 1,156 patients with hypertension were included in the study. Five patients were excluded due to incomplete serum drug measurements and irregularities. Of the n = 1,151 remaining patients, n = 79 patients (6.9%) were found to be non-adherent by serum drug concentration measurements, and n = 1,072 patients (93.1%) were adherent (Figure 1).
The non-adherent patients were younger (56.9 vs. 63.7 years, p < 0.001), and accordingly, the time since diagnosis of hypertension was shorter. There was no difference in adherence status between genders. Fewer non-adherent patients were of European ethnicity (82.9 vs. 95.8%, p < 0.001) (Table 2). They had significantly higher office BP and ABPM, both systolic and diastolic, compared to the adherent patients. Mean ambulatory heart rate was also significantly increased. Furthermore, non-adherent patients more often had uncontrolled hypertension compared to adherent patients (68.4 vs. 52.5%, p = 0.007).
Characteristics of study groups
| Variable | Adherent(n = 1,072) | Non-adherent(n = 79) | p-value |
|---|---|---|---|
| Female gender, n (%) | 461 (43.0) | 32 (40.5) | 0.665 |
| Age, years (SD) | 63.7 (11.0) | 56.9 (12.8) | < 0.001 |
| European ethnicitya, n (%) | 927 (95.8) | 58 (82.9) | < 0.001 |
| Body mass index, kg/m2 (SD) | 29.7 (5.3) | 29.1 (5.0) | 0.315 |
| Time since diagnosis of hypertension, years (SD) | 15.5 (11.4) | 11.4 (9.0) | 0.002 |
| Smoking habits, n (%) | |||
| Never-smokers | 456 (42.5) | 32 (40.5) | 0.724 |
| Previous smokers | 500 (46.7) | 31 (39.2) | 0.012 |
| Smokers | 116 (10.8) | 16 (20.3) | 0.011 |
| BPs and heart rate | |||
| Office systolic BP, mmHg (SD) | 143.2 (18.6) | 150.4 (22.0) | 0.001 |
| Office diastolic BP, mmHg (SD) | 82.0 (11.3) | 91.4 (14.8) | < 0.001 |
| Office heart rate, beats/min (SD) | 68.4 (12.6) | 70.4 (11.7) | 0.168 |
| Ambulatory daytime systolic BP, mmHg (SD) | 136.4 (14.6) | 142.9 (16.4) | < 0.001 |
| Ambulatory daytime diastolic BP, mmHg (SD) | 79.6 (9.5) | 86.6 (12.4) | < 0.001 |
| Ambulatory daytime heart rate, beats/min (SD) | 70.4 (10.5) | 74.7 (10.9) | < 0.001 |
| Uncontrolled hypertensionb, n (%) | 553 (52.5) | 52 (68.4) | 0.007 |
| Orthostatic hypotension, n (%) | 52 (4.9) | 4 (5.1) | 0.79 |
| Biochemical and urine analyses (SD) | |||
| Serum total cholesterol, mmol/L | 4.9 (1.2) | 4.7 (0.9) | 0.037 |
| Serum HDL, mmol/L | 1.5 (0.4) | 1.4 (0.5) | 0.501 |
| Serum LDL, mmol/L | 3.1 (1.1) | 3.0 (0.9) | 0.318 |
| Serum triglycerides, mmol/L | 1.8 (1.1) | 1.6 (1.0) | 0.071 |
| Serum creatinine, µmol/L | 81.0 (23.7) | 77.5 (25.4) | 0.236 |
| eGFR, mL/min/1.73 m2 | 80.4 (17.6) | 87.5 (19.0) | < 0.001 |
| Urine A/C ratio, mg/mmol | 6.6 (29.3) | 8.3 (37.9) | 0.644 |
| HbA1c, mmol/mol | 40.3 (9.1) | 39.4 (11.3) | 0.432 |
Results are reported as n (%) or mean (SD), p-value denotes differences between the adherent and the non-adherent group. SD: standard deviation; BP: blood pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein; eGFR: estimated glomerular filtration rate; A/C: albumin/creatinine; HbA1c: hemoglobin A1c. a For the variable European ethnicity, there were in total n = 113 patients with missing data; b for the variable uncontrolled hypertension, n = 22 patients had missing data
Figure 2 shows the age distributions of the non-adherent and adherent patients. The bar charts reveal that non-adherence is present in all age groups. However, the age groups with the highest percentage of non-adherence were 18–39 years and 40–59 years. Within the age group 18–39 years, n = 7 of 33 included patients were non-adherent (21.2%). The corresponding prevalence in the age group 40–59 years was 11.8%.
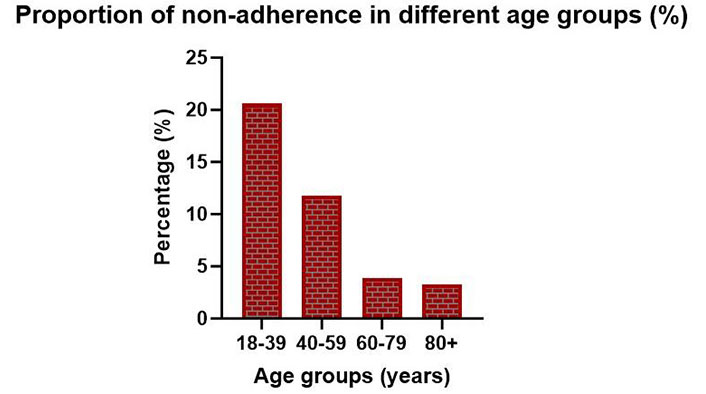
Bar chart showing the proportion of non-adherence in different age groups of included patients
Medications
Non-adherent patients were prescribed more antihypertensive pills (p = 0.006) than adherent patients. However, the number of prescribed concomitant pills was not significantly different (Table 3). Further, the non-adherent patients were more prone to use only single-agent pills and fewer combination pills compared to adherent patients. The most used drug was angiotensin receptor blocker (ARB), with calcium channel blockers (CCBs) and diuretics as the second and third most used antihypertensive drugs in the study (Figure 3). There were significant differences in the prescription of the major drug classes between the non-adherent and adherent patients. Non-adherent patients were more often prescribed CCBs and angiotensin converting enzyme inhibitors (ACEis), whilst adherent patients had a higher frequency of ARBs and diuretics.
Current medications in the study groups
| Variable | Adherent(n = 1,072) | Non-adherent(n = 79) | p-value |
|---|---|---|---|
| Medication overview, mean (SD) | |||
| Number of prescribed daily antihypertensive pills | 2.26 (1.2) | 2.65 (1.4) | 0.006 |
| Number of prescribed antihypertensive agents | 2.89 (0.9) | 3.10 (1.1) | 0.058 |
| Number of prescribed concomitant pills | 2.46 (2.4) | 2.65 (3.2) | 0.499 |
| Total number of prescribed daily pillsa | 4.71 (2.9) | 5.29 (3.8) | 0.099 |
| Antihypertensive medications, n (%) | |||
| ACEis | 147 (13.7) | 19 (24.1) | 0.012 |
| ACEi single-agent drug | 86 (8.0) | 14 (17.7) | 0.006 |
| ACEi combination with hydrochlorothiazide | 51 (4.8) | 4 (5.1) | 0.787 |
| ACEi combination with CCB | 10 (0.9) | 1 (1.3) | 0.544 |
| ARBs | 894 (83.4) | 58 (73.4) | 0.024 |
| ARB single-agent drug | 242 (22.6) | 19 (24.1) | 0.762 |
| ARB combination with hydrochlorothiazide | 427 (39.8) | 22 (27.8) | 0.035 |
| ARB combination with CCB | 81 (7.6) | 7 (8.9) | 0.660 |
| ARB triple combination (ARB + CCB + thiazide) | 144 (13.4) | 10 (12.7) | 0.863 |
| CCBs | 740 (69.0) | 64 (81.0) | 0.025 |
| CCB single-agent drug | 505 (47.1) | 46 (58.2) | 0.056 |
| Diureticsb | 694 (64.7) | 41 (51.9) | 0.022 |
| Aldosterone antagonists | 57 (5.3) | 6 (7.6) | 0.390 |
| Beta-blockers | 388 (36.2) | 33 (41.8) | 0.321 |
| Alpha-adrenoreceptor blockers | 36 (3.4) | 5 (6.3) | 0.169 |
| Centrally acting sympathicolytics | 47 (4.4) | 4 (5.1) | 0.777 |
| Antihypertensive agents in single-agent or combination pills, n (%) | |||
| Only single-agent pills | 348 (32.5) | 34 (43.0) | 0.054 |
| Only 1 antihypertensive pill | 296 (27.6) | 11 (13.9) | 0.008 |
| Fixed-dose combination pill, one or more | 724 (67.5) | 45 (57.0) | 0.054 |
| Triple combination pill | 144 (13.4) | 10 (12.7) | 0.863 |
| Selected concomitant medications, n (%) | |||
| Lipid-lowering drugsc | 523 (48.8) | 36 (45.6) | 0.763 |
| Anti-diabetic drugsd | 167 (15.6) | 12 (15.2) | 0.972 |
| Anti-coagulantse | 386 (36.0) | 31 (39.2) | 0.391 |
Results are reported as n (%) or mean (SD), p-value denotes differences between the adherent and the non-adherent group. SD: standard deviation; ACEis: angiotensin converting enzyme inhibitors; CCB: calcium channel blocker; ARBs: angiotensin receptor blockers. a All antihypertensive pills + all concomitant pills; b diuretics include loop diuretics and thiazides; c lipid-lowering drugs include all cholesterol-lowering drugs; d antidiabetic drugs include oral antidiabetics and insulin; e anticoagulants include antiplatelet drugs and direct oral anticoagulants
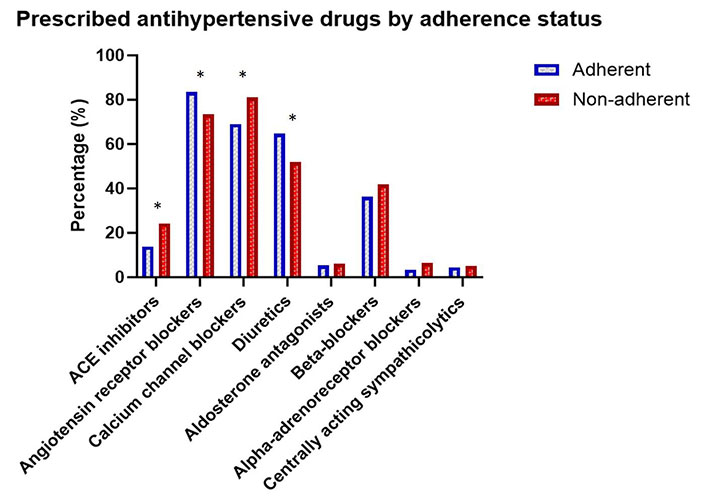
Bar chart showing the percentages of prescribed antihypertensive drugs among non-adherent and adherent patients. ACE: angiotensin converting enzyme. *: p < 0.05 for comparison of drug prescriptions between non-adherent and adherent patients
It can be seen from Table 3 that ACEis and CCBs have higher % in the non-adherent patient group with lower “n”. Further, the numbers of patients taking other antihypertensive drugs are overall lower than for ARBs and thus there was better statistical power for ARB in the subsequent multivariate analyses. Regarding the use of combination drugs, the non-adherent group used more frequently ACEis and CCBs in monotherapy compared to the adherent group, whilst the adherent group had significantly more use of ARB in combination with hydrochlorothiazide.
Biochemical analyses
As shown in Table 2, there were no large differences in selected biomarkers between non-adherent and adherent patients, except that non-adherent patients had moderately increased eGFR (87.5 vs. 80.4 mL/min/1.73 m2, p < 0.001) in line with their younger age and hypertension of shorter duration.
Patients with diabetes
We did not find that patients with diabetes (n = 195, 16.9%) tended to be more, or less, adherent than other patients (p = 0.309). Patients with diabetes did not have significantly higher systolic BP than patients without diabetes (144.4 vs. 143.6 mmHg, p = 0.585). However, they had higher serum creatinine (83.7 vs. 79.9 µmol/L, p = 0.044) and a lower eGFR (79.6 vs. 81.2 mL/min/1.73 m2).
Characteristics associated with non-adherence
The final statistical multivariate model included four variables (Table 4). The factor strongest associated with non-adherence was age. We categorized age into quartiles and computed a dichotomous variable with the youngest quartile as the reference category vs. quartiles 2 to 4. The youngest age quartile had nearly four times higher odds of being non-adherent compared to the older three quartiles. The number of antihypertensive pills increased the risk of non-adherence by 26% for each pill. Non-Europeans had a 3.3 times higher risk of being non-adherent than Europeans. Patients who used ARBs were more likely to be adherent than patients not using ARBs. Interaction analyses did not demonstrate any significant interactions.
Variables associated with non-adherence
| Variable | B | Standard error | p-value | Odds ratio (95% CI) |
|---|---|---|---|---|
| Age (dichotomized)* | 1.31 | 0.26 | < 0.001 | 3.72 (2.23–6.22) |
| Non-European ethnicity | 1.18 | 0.37 | 0.002 | 3.26 (1.57–6.76) |
| Number of AH pills | 0.23 | 0.10 | 0.015 | 1.26 (1.05–1.52) |
| Use of ARB | –0.60 | 0.30 | 0.046 | 0.55 (0.31–0.99) |
* Youngest quartile (Q1) compared to other three quartiles (Q2–Q4) dichotomized. Use of ARB; compared to other antihypertensive medications dichotomized. B: beta; CI: confidence intervals; AH: antihypertensive; ARB: angiotensin receptor blocker
Higher BP was also significantly associated with non-adherence but might as likely be a result of non-adherence as a predictor for non-adherence and was therefore not included in the final statistical multivariate model. Most study participants were non-smokers (never smokers or previous smokers) (Table 2). Though there were some moderate differences in fractions of active smokers, the numbers were rather small, and smoking status did not make it to the final statistical multivariate model of characteristics that were associated with drug adherence shown in Table 4.
Discussion
We investigated n = 1,151 patients in a cross-sectional study of BP control and drug adherence. Of these patients, n = 1,072 patients were identified as adherent to their antihypertensive drugs, and n = 79 patients (6.9%) were identified as non-adherent by serum drug concentration measurements. We found the non-adherent patients to be younger, with higher systolic and diastolic office BP, and less likely to be of European ethnicity. Characteristics associated with non-adherence in logistic regression analysis were age, number of antihypertensive pills, ethnicity, and the use of ARBs in favor of better adherence.
In our cross-sectional study, we found 6.9% of patients only to be non-adherent, which is lower than the prevalence found in other studies of drug adherence in hypertension [5, 7]. A main reason for this low prevalence might be due to selection of patients in the inclusion process [15]. Patients had to agree to be included in the study, and it is likely that only motivated patients would agree. Also, most of the patients were included from GPs, and we assume that patients regularly visiting their GP are more drug adherent than patients who rarely visit their physician.
The Hawthorne effect may have influenced the study results. The Hawthorne effect indicates a shift in patients’ behavior when being observed, and it is reported to influence adherence in other studies [16]. Participating in a hypertension study may have reminded people to take their medications as prescribed. However, the Hawthorne effects may have influenced hypertension patients also in all other studies of antihypertensive drug adherence. In the present study we deliberately did not focus on drug adherence and serum analysis of drug adherence was not mentioned in the written informed consent signed by the patients—a procedure approved by the Ethical Committee.
The low number of non-adherent patients could also be influenced by a strict definition of non-adherence; patients needed to refrain from their antihypertensive medications for up to several days subsequently for the serum concentration of their medications to fall below the cut-offs defining non-adherence [14].
We are unsure why younger patients were less adherent than older patients, but it might be related to less tolerability of side effects in younger patients, or to a lower level of confidence in their physicians’ authority. Non-Europeans were also less likely to be adherent. This might be caused by a language barrier, despite inclusion criteria included “fluency in Norwegian language”, or perhaps a lack of trust in the national healthcare system. The findings of patients of European ethnicity and older patients being more adherent to antihypertensive medications have also been found in similar studies by others [17].
We are currently not able to analyze the socioeconomic status (i.e., income, education, and occupation) of our study participants in detail though we believe that such variables plus culture and traditions, and disease history, most likely explain the lower drug adherence of the non-European patients. With this, we mean that European ethnicity is historically better attached to the national health service including more long-lasting treatment and trust in their family physicians or GPs.
A higher number of antihypertensive pills could make it more difficult to remember to take all the medication as prescribed, but it could also be a result of the doctor prescribing more antihypertensive medications when patients seemingly do not have the expected BP lowering effect due to non-adherence. Patients using ARBs were more likely to be adherent. ARBs are known to cause few or no side effects as compared to placebo, with the lowest discontinuation rate of all antihypertensive agents [18].
Differences in persistence between different groups of antihypertensive drugs could not be detected in our study. Similar findings were previously reported in a population-based Swedish study [19]. The Swedish investigators identified [19], from their national Primary Care Cardiovascular Database, hypertensive patients (n = 4,997) with mean age of 60 ± 12 years in men and 63 ± 13 years in women. Out of these, 95 (2%) filled their first prescription for fixed combination therapy and 4,902 (98%) for monotherapy, including ACEis (37%), ARBs (4%), beta-blockers (21%), CCBs (8%), and diuretics (28%). Persistence in the initial drug class was 57% after 1 year and 43% after 2 years. There were no differences in persistence between diuretics and any of the other antihypertensive drug classes, after adjustment for confounders [19]. Discontinuation (all adjusted) was more common in men (p = 0.004), younger patients (p < 0.001), those with mild systolic BP elevation (p < 0.001), and patients born outside the Nordic countries (p < 0.001). Among 1,295 patients who switched drug class after their first prescription, only 21% had a BP recorded before the switch occurred; and out of them 69% still had high BP. This Swedish study [19] of patients with hypertension in primary healthcare on newly initiated drug therapy suggested that persistence is similar for any antihypertensive treatment when compared with diuretics, provided that confounding factors, often overlooked in previous studies, are properly adjusted for.
The Swedish study [19] was, however, different from our study in many aspects. They detected patients in a registry being prescribed first time antihypertensive medications in 2006–2007 and 4% of patients only were prescribed ARBs and 2% only fixed combination therapy. The Swedish study investigated 2-year drug persistence [19] at variance from our study of actual drug adherence. Also, the Swedish study considered a maximum of 30 days of medication gap to be persistent, while in our study the patients needed to have a measurable blood-level of the drug to be considered adherent. A main difference was thus the investigational method used to study adherence or persistence to medication as our study comprised serum measurements of the 23 most commonly used antihypertensive medications. The Swedish study on the other side analyzed all filled prescriptions of antihypertensive drugs prescribed to the cohort within a 2-year period. Assessing medication persistence through patients filling their prescriptions might have led to overestimation of persistence which was considered a limitation of the Swedish study [19]. Further, a registry study like the Swedish [19] did not include patient contact by the investigators or serum blood measurements, and it was retrospective in nature.
Strengths and limitations
The low prevalence of non-adherent patients is a limitation by itself in our study. It also makes it difficult to do subgroup analysis with any meaningful statistical power. In this and in other studies, ethnicity has been an important factor for adherence. Our included patients do not reflect the diversity of the Norwegian society, with an overall non-European ethnicity of approximately 20%; maybe a higher number of non-European patients would have yielded different adherence levels.
The recruitment procedures for patients to be investigated, with or without potential biases, are likely a key to explaining various fractions of patients with reduced adherence to antihypertensive drugs. In the present study, we made no focus whatsoever on drug adherence or persistence, without any mention of drug analyses in blood samples neither orally nor in written informed consent (signed on the same day as blood sampling). However, we had to advertise for hypertensive patients to volunteer for the study; patients who were on stable treatment with ≥ 2 antihypertensive drugs. This activity might of course by itself have brought attention to the drug treatment and improved the drug adherence of the study participants.
Our study also had strengths. Investigators were highly trained doctors and study personnel in the study clinics, all located within a university hospital. The number of investigated antihypertensive patients was high in the context of such chemical adherence testing, even if there was a relatively low number of non-adherent patients as earlier mentioned. Finally, we used a method to investigate drugs for non-adherence that is reliable, with low risk of false positive or negative test results [14].
Conclusions
We found fewer non-adherent hypertensive patients (6.9%) than we expected. However, we investigated a population-based sample of hypertensive patients on ≥ 2 antihypertensive drugs; patients who had previously received limited attention in this context. Our results on characteristics of adherence are in line with but also extend findings from other studies. As we see it emphasis should be put on information and close follow-up to ensure adherence especially in young patients and in patients of non-European ethnicity. The use of ARBs and single-pill combinations seems to increase adherence and could be preferred from this point of view when prescribing antihypertensive medication.
Abbreviations
| ABPM: | ambulatory blood pressure measurement |
| ACEis: | angiotensin converting enzyme inhibitors |
| ARB: | angiotensin receptor blocker |
| BP: | blood pressure |
| CCBs: | calcium channel blockers |
| eGFR: | estimated glomerular filtration rate |
| GPs: | general practitioners |
| MS/MS: | tandem mass spectrometry |
| UHPLC: | ultra-high-pressure liquid chromatography |
Declarations
Acknowledgments
The authors thank the patients who participated in the study and the physicians who referred them.
Author contributions
EO: Investigation, Formal analysis, Writing—original draft. LVH and KMB: Investigation, Data curation. SR, AA, and OUB: Investigation. VNK: Investigation, Data curation, Validation. KL: Supervision. MDS, RM, EG, SEK, and AH: Project administration, Writing—review & editing. MR: Project administration. MSO: Methodology, Investigation. ACKL: Project administration, Data curation, Formal Analysis, Writing—review & editing, Funding acquisition. CLS: Project administration, Formal Analysis, Writing—review & editing, Supervision, Funding acquisition.
Conflicts of interest
Marit D. Solbu has received honoraria from AstraZeneca, Novartis, Bayer and Boehringer-Ingelheim. Sverre E. Kjeldsen reports lecture honoraria from Emcure, Getz, Glenmark, J.B. Pharma, Merck Healthcare KGaA, and Vector-Intas in the past 3 years. Sverre E. Kjeldsen is the Guest Editor of Exploration of Medicine but had no involvement in the decision-making or the review process of this manuscript. Aud Høieggen reports lecture honoraria from AstraZeneca. The other authors declare that there are no conflicts of interest.
Ethical approval
The study was approved by the Regional Ethical Committee of the UiT Arctic University in Norway, Tromsø, Norway (approval no. 2017/804/REK Nord) and complies with the Declaration of Helsinki.
Consent to participate
All participants signed written informed consent.
Consent to publication
Not applicable.
Availability of data and materials
Requests to the corresponding author in dire need.
Funding
The study was supported by grants from the Research Council of Norway [273563], the South-East Norwegian Regional Health Authority, the University of Oslo, Oslo University Hospital, Ullevaal, and the North Norwegian Regional Health Authority. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.