Abstract
Aim:
Breast cancer (BC) is the leading cause of female cancer-related death worldwide. The high incidence of BC has sparked interest in the viral agents role in its development. Identifying co-infection involving potential oncogenic viruses, such as human papillomavirus (HPV), Epstein-Barr virus (EBV), mouse mammary tumor virus (MMTV), and Simian virus 40 (SV40), may improve early detection and treatment strategies of BC. However, the involvement of these viral co-infections in invasive breast cancer (IBC) has not been elucidated.
Methods:
To investigate this eventual co-infection, screening of viral DNAs isolated from 120 fresh IBC tissue biopsies was performed using a polymerase chain reaction. Statistical analysis were conducted to assess the correlation between viral prevalence and IBC clinicopathological features.
Results:
Our findings revealed the prevalence of EBV (67.1%), HPV (45.7%), MMTV (72.9%), and SV40 (22.9%) in IBC samples. Co-infection rates were as follows: EBV/HPV (17.14%), EBV/MMTV (22.86%), EBV/SV40 (4.28%), and HPV/MMTV (11.43%). Triple infection with EBV, HPV, and MMTV was observed in 5.71% of cases. Statistically significant associations were identified between: EBV and histological grade, tumour size and stage, and progesterone receptor (PR) and estrogen receptor (ER) status; HPV and histological grade, tumour stage, and PR status; MMTV and histological type, as well as PR and ER status; and SV40 and histological grade and PR status.
Conclusions:
Although the prevalence of HPV, EBV, MMTV, and SV40 coinfection in Moroccan IBC patients is low, their potential synergistic role in breast carcinogenesis needs to be further investigated, in order to identify new etiological factors for BC.
Keywords
Invasive breast cancer, HPV, EBV, MMTV, SV40, coinfection, viral etiology, PCRIntroduction
Breast cancer (BC) is the most common cancer in women worldwide with alarming incidence and mortality rates. The etiology of BC is multifactorial, involving host genetic factors, viral infections, environmental factors, and hormonal factors related to estrogen and progesterone. Namely, prolonged use of oral contraceptives, early age of menarche, late age of menopause, and postmenopausal obesity [1]. However, there are other co-factors coupled to hormone-sensitive viruses, which induce malignant transformation of virally infected breast cells. Among the main oncoviral agents responsible for the development of BC are human papillomavirus (HPV), Epstein-Barr virus (EBV), mouse mammary tumor virus (MMTV), and Simian virus 40 (SV40), but there is no conclusive evidence for a causal role of co-infection with these viruses in BC [2–4].
HPV and breast cancer
HPVs are small non-enveloped particles of viruses containing a double-stranded DNA genome of approximately 8 kb [5]. They encompass 200 genotypes, some of which infect mucosal and skin epithelial cells, and about 50 genotypes cause genitourinary infections [6], leading to the development of specific cancers. HPV is the leading common cause of sexually transmitted genital infections worldwide and the main causative factor of cervical cancer, one of the most common cancers in women [7]. In Morocco, it is the second most common cancer in women after BC [8].
HPV genotypes are classified according to their oncogenic potential: high-risk oncogenic HPV and low-risk oncogenic HPV. Numerous studies have shown that approximately 99.7% of cervical cancers are associated with high-risk oncogenic HPVs infections, particularly HPV 16 and 18 [9]. These two are frequently observed in high-grade intraepithelial lesions and neoplasia [10]. Thus, cell transformation and maintenance of the malignant phenotype are linked in particular to the expression of the two viral oncoproteins E6 and E7 of HPV as well as to the persistence of HPV infection, which can potentially progress to precancerous lesions and then to invasive malignancies, leading to carcinogenesis [7].
The oncogenic HPV infections are also associated with other types of cancer, such as cancer of the penis, anus, vulva, vagina, neck, head, oropharynx [11, 12]. Moreover, several studies have highlighted the involvement of HPV in BC tissues and cell lines. Shortly after the discovery of the association between HPV and BC, evidence of the frequent involvement of HPV 16 in multiple invasive and metastatic BC was reported [13, 14]. HPV genotypes 16, 18, and 33 have also been identified in BC from very different populations [15]. Thus, it has been proposed that infection with high-risk oncogenic HPVs may drive cell invasion and metastasis in BC via Id-1, a family of helix-loop-helix transcription factors [14, 16].
EBV and breast cancer
EBV is a ubiquitous human virus, infecting 90% of the population, and is linked to several human malignancies, including BC [17]. EBV is a γ-herpesvirus whose genome, 184 kb in length of DNA, encodes about 100 genes [18].
The Horiuchi team identified EBV genetic material for the first time in Japanese women with BC in 1994 [19]. To date, more than 50 studies on EBV and BC have followed. EBV has been reported to be involved in the development of invasive breast cancer (IBC) [20]. Fina and coworkers described a predominance of the EBV1 subtype in BC samples [21]. The link between BC and EBV provides information not only on the viral etiology of BC, but also on the early diagnosis, therapy, and prevention of this disease.
Moreover, a current study indicates that EBV and HPV coexist in 32% of the same BC samples in women [2]. In the same study, viral DNA for EBV, HPV, and MMTV was detected in more than 50% of Australian BC cases [2].
MMTV and breast cancer
MMTV is a betaretrovirus, known as a milk-borne agent that causes mouse mammary cancer [22]. 70% of the complete MMTV-like virus genome found, in human BC tissues was sequenced and showed 91–99% homology with MMTV from murine mammary tumors [23, 24]. Although the MMTV gene sequence has been detected in human BC, its role in humans is still disputable [22]. However, evidence from Stewart and Chen (2022) [25] suggests that MMTV zoonosis may contribute to the geographic variation in BC incidence. A recent study reported the identification of MMTV, EBV, and HPV in the DNA of 50 fresh IBC samples, and the presence of more than one viral type in the same IBC sample [2].
SV40 and breast cancer
The SV40 virus has been added to the growing list of viruses suspected of being associated with BC. It is a monkey-origin polyomavirus, involved in several human tumors. SV40 carcinogenesis is based on the expression of the large viral antigen T (Tag) oncoprotein, which induces progression and apoptosis of infected cells by promoting the IGF-I (insulin-like growth factor-1) receptor, c-MET, and MAPK (mitogen-activated protein kinase) signaling pathways, as well as by inhibiting the two tumor suppressors p53 and pRB (retinoblastoma protein) [26].
In 2002, Wong et al. [3] described novel associations between HPV, EBV, and SV40 in some human cancers, including the association of EBV and BC, but no relationship between SV40 and BC was reported. However, testing for SV40 in 109 BC specimens revealed the presence of SV40 Tag sequences in 22% of the cases [27].
As well, Hachana et al. [28] identified SV40-positive DNA in BC tissue with a low rate. The prevalence of SV40 antibodies in serum samples from women with the combination is elaborate and substantial but cannot be considered conclusive.
Therefore, to investigate the obvious presence or absence of viral coinfection in IBC, 120 IBC cases were screened for HPV, EBV, MMTV, and SV40 by molecular detection of DNA from each virus by polymerase chain reaction (PCR). In addition, statistical correlation analysis of the clinicopathologic characteristics of the patients and the candidate viruses was performed.
Materials and methods
Breast cancer patients and specimens
Our study was performed on 120 fresh biopsies, 70 tumor tissue samples and 50 matched normal tissue samples, obtained from patients with histopathologically confirmed IBC after surgery in the Onco-Gynecology Department of the Mohammed IV Oncology Center, Casablanca, Morocco, over two years from January 2019 to December 2021. After collection, biopsies were immediately stored at –80°C until use. Patients who underwent chemotherapy and/or radiotherapy were excluded.
The Ethics Committee for Biomedical Research of the Faculty of Medicine and Pharmacy of Casablanca, Morocco (Reference 3/2018 on 30.04.2018) approved the study protocol. In accordance with the ethical rules, free and informed consent was acquired from all patients who were included.
Clinicopathological parameters of the recruited cases were collected according to STROCSS guidelines to enhance the quality and relevance of the information acquired [29].
IBC patients clinicopathological characteristics are described in Table 1. The mean age of the patients was 45 (± 10.99) years (range: 29–78 years). The most common age range was ≥ 40 years, accounting for 74.3% of cases. Regarding histological findings, 38 patients (54.3%) had grade I or II, and 32 (45.7%) had grade III. 65 patients (92.9%) had invasive ductal carcinoma, and only five (7.1%) had invasive lobular carcinoma. In 52 cases (74.3%), the tumor size was superior to 5 cm. The majority of patients (57; 81.4%) were in stages T1 and T2. Regarding hormonal factors, 39 patients (55.7%) showed a positive progesterone receptor (PR) and 29 (41.4%) showed a positive estrogen receptor (ER).
Clinicopathological features of invasive breast cancer patients (n = 70)
| Features | n | Percentage (%) |
|---|---|---|
| Age at diagnosis (years): mean age = 45 (± 10.99) | ||
| < 40 | 18 | 25.7 |
| ≥ 40 | 52 | 74.3 |
| Histological grade | ||
| I–II | 38 | 54.3 |
| III | 32 | 45.7 |
| Histological type | ||
| Invasive ductal | 65 | 92.9 |
| Invasive lobular | 5 | 7.1 |
| Tumor size (in cm) | ||
| ≤ 5 | 18 | 25.7 |
| > 5 | 52 | 74.3 |
| Tumor stage | ||
| T1–T2 | 57 | 81.4 |
| T3–T4 | 13 | 18.6 |
| Progesterone receptor (PR) | ||
| Positive | 39 | 55.7 |
| Negative | 31 | 44.3 |
| Estrogen receptor (ER) | ||
| Positive | 29 | 41.4 |
| Negative | 41 | 58.6 |
n: number of patients
DNA extraction
DNA was extracted from frozen tissue subsamples of ≤ 25 mg taken from our IBC biopsies using the Purelink Invitrogen® Genomic DNA Minikit (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. DNA extracts were analyzed directly or stored at –20°C until use.
Qualitative and quantitative DNA analysis
The NanoDrop 2000 spectrophotometer (Technologies, Wilmington, DE, USA) was used to evaluate the concentration and purity of the extracted DNA. To assess the quality of the extraction, the integrity of the DNA extracts and the absence of PCR inhibitors, the β-globin gene used as a reference housekeeping gene was amplified by PCR using the primers PCO4 and GH20 (Table 2) as described previously [30].
Primers sequences used for PCR amplification
| Gene | Primers sequences (5–3) | Tm (°C) | PCR product size (bp) |
|---|---|---|---|
| β-globin | GH20-F 5'-GAAGAGCCAAGGACAGGTAC-3' | 54 | 268 |
| PC04-R 5'-CAACTTCATCCACGTTCACC-3' | |||
| HPV | MY09-F 5'-CGTCCMARRGGAWACTGATC-3' | 55 | 450 |
| MY11-R 5'-GCMCAGGGWCATAAYAATGG-3' | |||
| GP5+-F 5'-TTTGTTACTGTGGTAGATACTAC-3' | 42 | 150 | |
| GP6+-R 5'-GAAAAATAAACTGTAAATCATATTC-3' | |||
| EBV | EBNA-2 OP-F 5'-GCGGGTGGAGGGAAAGG-3' | 58 | 168 |
| EBNA-2 OP-R 5'-GTCAGCCAAGGGACGCG-3' | |||
| EBNA-2 IP-F 5'-AGGCTGCCCACCCTGAGGAT-3' | 66 | 168 | |
| EBNA-2 IP-R 5'-GCCACCTGGCAGCCCTAAAG-3' | |||
| SV40 | SVTAGP1-F 5'-TTAGCAATTCTGAAGGAAAGTCCTTG-3' | 52 | 126 |
| SVTAGP3-F 5'-ACCTGTTTTGCTCAGAAG-3' | |||
| MMTV | MMTV1-F 5'-TGCGCCTTCCCTGACCAAGGG-3' | 54 | 356 |
| MMTV2-R 5'-GTAACACAGGCAGATGTAGG-3' |
F: forward primer; R: reverse primer; PCR: polymerase chain reaction; Tm: melting temperature; HPV: human papillomavirus; EBV: Epstein-Barr virus; MMTV: mouse mammary tumor virus; SV40: Simian virus 40. M = A + C, R = A + G, W= A + T, Y = C + T
Viral DNA detection
DNA extracts from IBC samples were screened for viral DNA of each HPV, EBV, MMTV, and SV40. This analysis was performed by PCR amplification, using specific primer pairs (Table 2). Gene sequences of HPV and EBV were amplified by nested PCR using appropriate consensus primer sets, as previously described [31, 32]. However, a single-PCR round was performed to amplify MMTV-like and SV40 viral DNA using specific primer sets, as previously described [33, 34].
Briefly, the PCR reaction mixture with a total volume of 25 µL consisted of 6.5 µL of ultrapure water, 2 µL of forward and reverse primers, and 12.5 µL of Taq PCR master mix (Vazyme Biotech, China) and, alternatively, 2 µL of concentrated DNA (100 ng/µL) for the first PCR, and 2 μL of the PCR product of the first reaction for nested-PCR. PCR amplification was performed on a Perkin Elmer 2400® thermal cycler (Scientific Support, Inc, Hayward, CA) under the following cycling conditions: initial denaturation at 94°C/10 min, 35 cycles of denaturation at 94°C/1 min, annealing of the primer at the corresponding melting temperature (Tm) for 1 min (Table 2), elongation at 72°C/1 min, followed by a final incubation at 72°C/10 min. A negative control containing the amplification reaction mixture without DNA was performed for each reaction.
To evaluate the outcome of each PCR, all PCR products were analyzed by 2% agarose gel electrophoresis at 70 V for 1.5 h, stained with ethidium bromide, then visualized under UV light (SERVA Electrophoresis GmbH, Heidelberg, Germany).
Statistical analysis
Statistical analysis of the data was generated using Jamovi software (The Jamovi project, 2021). The correlation between DNA-positive viruses and IBC cases and the clinico-pathologic parameters was carried out by Chi-square test or Fisher’s exact test (when one of the theoretical numbers is less than 5). The difference was considered statistically significant when the P < 0.05.
Results
Oncoviral DNA prevalence
In the present study, the β-globin gene was amplified in all 120 specimens (70 IBCs and 50 matched normal tissues), and the entire set of DNA samples was therefore suitable for further analysis. Our findings show that the prevalence of viral DNA sequences was significantly higher in IBC than in matched normal samples. Viral infection was detected in 61/70 (87.1%) of IBC tissues and 10/50 (20%) of matched normal tissues. Single and nested-PCR amplification of viral DNA sequences of each virus, namely EBV, HPV, MMTV, and SV40 revealed the detection of EBV DNA in (47/70; 67.1%) of IBC cases, HPV DNA in (32/70; 45.7%), MMTV DNA in (51/70; 72.9%), and SV40 DNA in (16/70; 22.9%). However, in the matched normal tissue samples, EBV, HPV, MMTV, and SV40 viral DNA was weakly detected in (6/50; 12%), (4/50; 8%), (8/50; 16%), and (0/50; 0%) of cases, respectively (Figure 1).
Statistical analysis of the PCR results of the cases showed a significant difference between the viral prevalence of all viruses enrolled in IBC tissues and matched normal tissues (P < 0.001) (Figure 1).
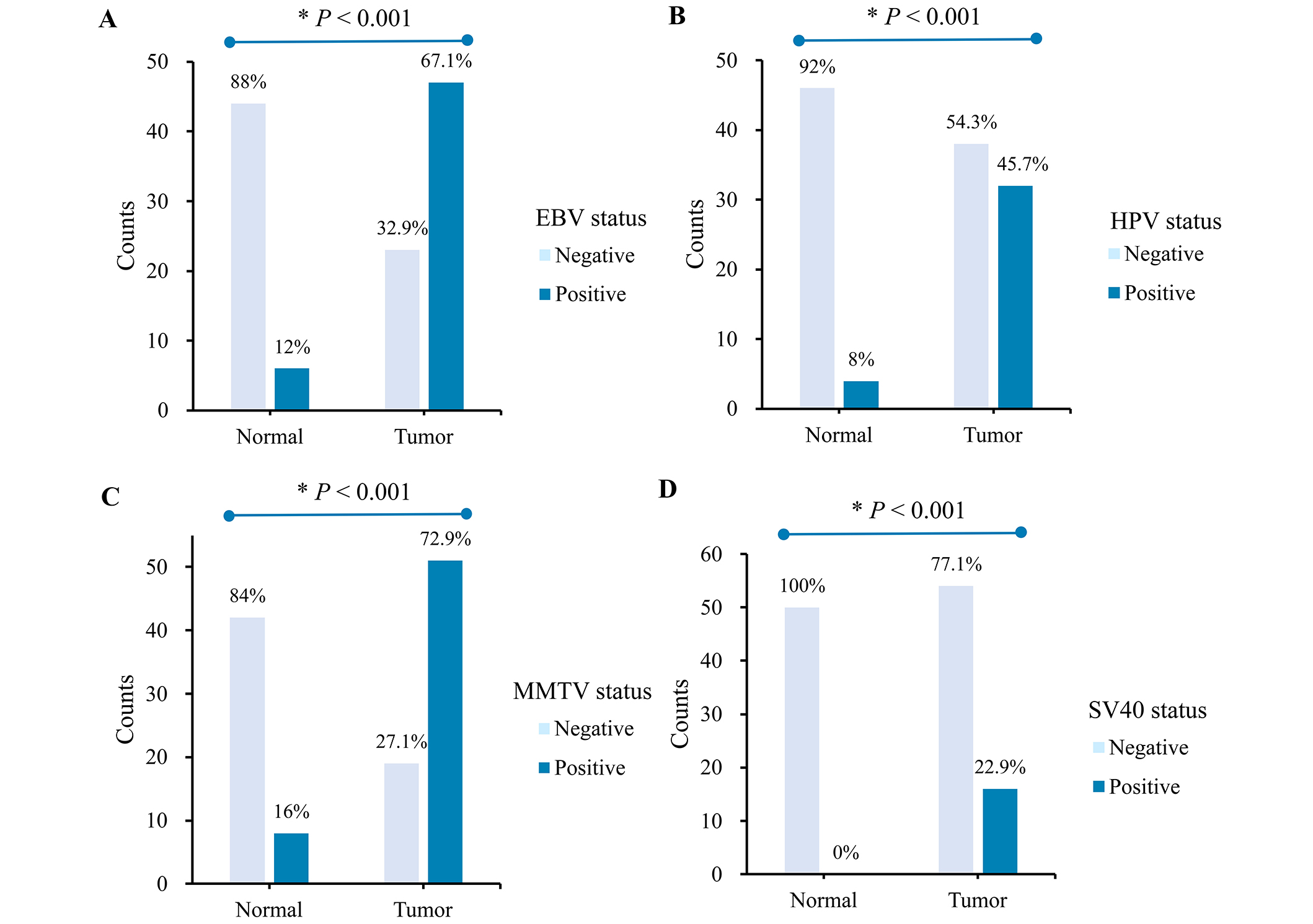
Prevalence of (A) EBV, (B) HPV, (C) MMTV, and (D) SV40 sequences in invasive breast cancer tumor tissues (n = 70) vs in matched normal tissues (n = 50). *P-value < 0.05 is considered significant. HPV: human papillomavirus; EBV: Epstein-Barr virus; MMTV: mouse mammary tumor virus; SV40: Simian virus 40
Association between EBV, HPV, MMTV, and SV40 infections and clinico-pathological features of IBC patients
Statistical analysis of the association between EBV, HPV, MMTV, and SV40 infection and clinico-pathological characteristics was evaluated and presented in Table 3. Regarding age at diagnosis, there is obviously no significant difference between the age and the IBC samples positive for all viruses studied. Statistical analysis clearly showed a significant difference between the presence of EBV viral DNA and histological grade (P = 0.022), tumor size and tumor stage (P < 0.001), positive PR (P = 0.003), and ER (P < 0.001). Thus, HPV infection is significantly associated with histological grade (P < 0.001), tumor stage (P = 0.004), and positive PR (P < 0.001). As well, MMTV infection is significantly associated with the type of invasive ductal carcinoma (P < 0.001), and the presence of PR and ER (P < 0.001). Finally, statistical analysis revealed that SV40 was also significantly associated with histological grade (P < 0.001), and positive PR (P = 0.023).
Distribution of EBV, HPV, MMTV, and SV40 positive cases according to clinicopathological features of IBC patients (n = 70)
| Features | Totaln (%) | EBV positive | HPV positive | MMTV positive | SV40 positive | ||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | P-value | n (%) | P-value | n (%) | P-value | n (%) | P-value | ||
| Age at diagnosis (years) | |||||||||
| < 40 | 18 (25.71) | 12 (66.7) | 0.960 | 5 (27.8) | 0.076 | 16 (88.9) | 0.123 | 6 (33.3) | 0.219 |
| ≥ 40 | 52 (74.29) | 35 (67.3) | 27 (51.9) | 35 (67.3) | 10 (19.2) | ||||
| Histological grade | |||||||||
| I–II | 38 (54.29) | 30 (78.9) | 0.022* | 26 (68.4) | < 0.001* | 30 (78.9) | 0.212 | 16 (42.1) | < 0.001* |
| III | 32 (45.71) | 17 (53.1) | 6 (18.8) | 21 (65.6) | 0 (0) | ||||
| Histological type | |||||||||
| Invasive ductal | 65 (92.86) | 45 (69.2) | 0.322 | 30 (46.2) | > 0.99 | 50 (76.9) | < 0.001* | 16 (24.6) | 0.207 |
| Invasive lobular | 5 (7.14) | 2 (40.0) | 2 (40.0) | 1 (20.0) | 0 (0) | ||||
| Tumor size (in cm) | |||||||||
| ≤ 5 | 18 (25.71) | 4 (22.2) | < 0.001* | 5 (27.8) | 0.076 | 15 (83.3) | 0.071 | 2 (11.1) | 0.209 |
| > 5 | 52 (74.29) | 43 (82.7) | 27 (51.9) | 36 (69.2) | 14 (26.9) | ||||
| Tumor stage | |||||||||
| T1–T2 | 57 (81.43) | 45 (78.9) | < 0.001* | 21 (36.8) | 0.004* | 41 (71.9) | > 0.99 | 13 (22.8) | > 0.99 |
| T3–T4 | 13 (18.57) | 2 (15.4) | 11 (84.6) | 10 (76.9) | 3 (23.1) | ||||
| Progesterone receptor (PR) | |||||||||
| Positive | 39 (55.71) | 32 (82.1) | 0.003* | 25 (64.1) | < 0.001* | 35 (89.7) | < 0.001* | 13 (33.3) | 0.023* |
| Negative | 31 (44.29) | 15 (48.4) | 7 (22.6) | 16 (51.6) | 3 (9.7) | ||||
| Estrogen receptor (ER) | |||||||||
| Positive | 29 (41.43) | 26 (89.7) | < 0.001* | 13 (44.8) | 0.901 | 13 (44.8) | < 0.001* | 7 (24.1) | 0.830 |
| Negative | 41 (58.57) | 21 (51.2) | 19 (46.3) | 38 (92.7) | 9 (22.0) | ||||
*P < 0.05 is considered significant. HPV: human papillomavirus; EBV: Epstein-Barr virus; MMTV: mouse mammary tumor virus; SV40: Simian virus 40; IBC: invasive breast cancer
Viral co-infection of EBV, HPV, MMTV and SV40 in IBC
Our findings demonstrated that EBV, HPV, MMTV, and SV40 viral sequences may all be present as individual or multiple viruses in IBC and in some normal breast tissues, as shown in Figure 2. The results showed that mono-infection with EBV, HPV, MMTV, or SV40 was detected in 21.43%, 11.43%, 32.86%, and 18.57% of cases respectively. However, co-infection of EBV and HPV was detected in 12 (17.14%), EBV and MMTV co-infection in 16 (22.86%), co-infection of EBV and SV40 in 3 (4.28%), and co-infection of HPV and MMTV in 8 (11.43%) of the IBC specimens (Figure 2). Triple infection with EBV, HPV, and MMTV was detected in only 4 (5.71%) of the samples. There was no viral coinfection of all viral sequences in any of the IBC samples. No significant statistical correlation was disclosed between the prevalence of co-infection and IBC disease findings.
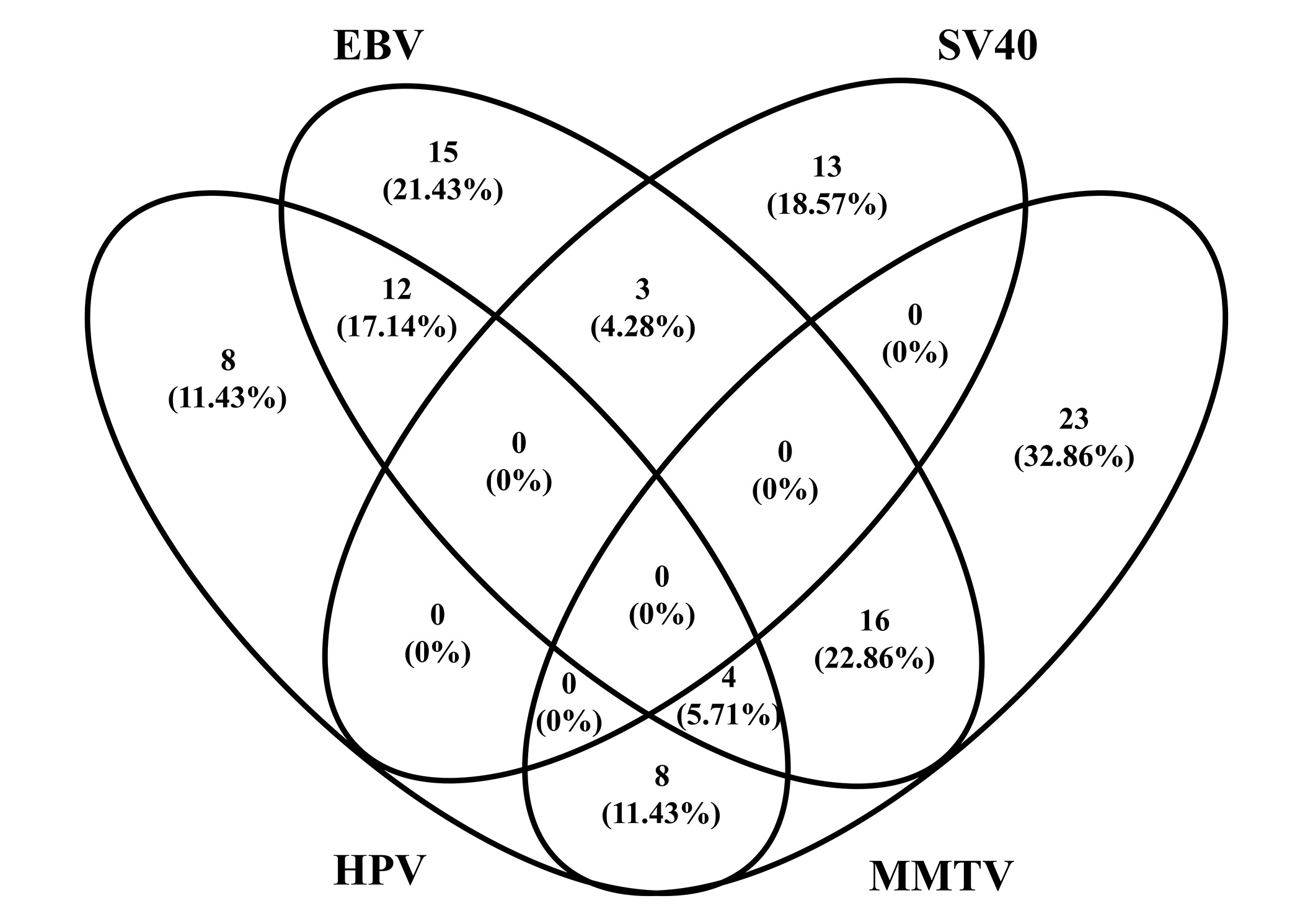
Diagram of the prevalence of EBV, HPV, MMTV, and SV40 co-infection in IBC tumor tissues. HPV: human papillomavirus; EBV: Epstein-Barr virus; MMTV: mouse mammary tumor virus; SV40: Simian virus 40; IBC: invasive breast cancer
Discussion
To the best of our knowledge, this is the first study to investigate the presence/co-presence of HPV, EBV, MMTV-like, and SV40 viruses in IBC tumor tissue in Morocco. Our study revealed that 87.1% of samples were positive for these oncoviruses.
Of particular interest, the prevalence of HPV in BC cases differed between countries worldwide. In Morocco, previous studies showed a prevalence of HPV DNA in around 25% of BC tumors [35], whereas our data showed a higher percentage of 45.7%. In fact, a recent assessment of HPV-related cancer incidence in Morocco, published by HPV center information, showed that the high level was attributed to BC at 63% compared to other types of non-cervical cancers [36]. This finding is consistent with the increasing frequency of HPV DNA in BC patients worldwide.
Various research on the investigation of HPV in BC revealed that 29% of BC samples carried high-risk HPV genotypes [37]. According to a recent study, in 855 cases of BC from the TCGA database, there were 30 (3.5%) low-risk and 20 (2.3%) high-risk HPV subtypes. The high-risk genotypes included HPV18 (48%), HPV113 (24%), HPV16 (10%), and HPV52 (10%) [38]. Most HPV types detected in BC studies belong to the high-risk HPV genotypes. However, a low-risk type, HPV6, was presented in co-infection with another high-risk type in a study of 700 samples from Thai women with BC [39]. Khan et al. [40] also detected HPV6 in 46% of BC samples of Japanese patients. de Villiers’ study reported that the most prevalent type in IBC was HPV11, followed by HPV6, while other types were detected at a low percentage, including HPV16, 23, 27, 57, 3, 15, 24, and 87 [41]. Moreover, several studies indicate that high-risk HPV types 16, 18, and 33 have been identified in BC patients from a diverse range of demographic groups. According to a PCR cohort research, HPV18 was found in 55% of IBC specimens, followed by HPV16 in 13% [38]. According to Hennig et al. [42], 46% of women with BC tested positive for HPV-16. Damin et al. [43] showed that Brazilian women with BC were mainly carriers of HPV16 (56%) and HPV18 (40%). 72 patients from China and Japan showed HPV33-positive BC [44]. Kan et al. [45] showed that 48% of BC tissues tested positive for HPV18. According to research by Fernandes et al. [46], HPV51 was found in 30.77% of BC samples, with HPV genotypes 18 and 33 following closely behind with 23.08%.
In fact, according to research on HPV16 DNA in BC samples, HPV16 is more frequently found in invasive and metastatic BC than in in-situ BC [14]. Id-1, a family of helix-loop-helix transcription factors, has been proposed as a potential mechanism through which high-risk HPV infection can cause cell invasion and metastasis in BC [14, 16].
On the other hand, a single EBV infection was detected in 67.1% of BC tissues and in 12% of normal tissues. Various EBV genes have been identified in BC in a wide range of countries. Richardson et al. [47] have summarized the results of these studies in a meta-analysis. The percentage of EBV positives detected by molecular methods in BC patients ranged from 24% to 100%, while in normal controls it ranged from 0% to 33%. However, the percentage is higher when tested by serology [48]. In Morocco, the high incidence of EBV infection in BC cases, as reported by Gihbid et al. [49] in a recent study, supports the idea that EBV infection may play a role in the promotion or progression of BC as an etiologic factor or cofactor in the oncogenic mechanism that raises the risk of various BC subtypes. The disparate outcomes might be brought on by sample source restrictions or geographic variations in the prevalence of EBV infection and biopsies. As a result, the detection rate varied according to the patient’s ethnicity, the study’s approach, and the sample’s demographics [50].
MMTV-like involvement in BC has also been evaluated. In our current study, viral sequences were detected in 72.9% of cases. Two previous Moroccan studies showed relatively independent frequencies of MMTV-like in BC (57.14% and 56%, respectively) [51, 52]. Indeed, MMTV-like infection has been shown to be strongly associated with the inflammatory phenotype of BC. Unfortunately, the inflammatory status of our sample was not investigated. Overall, the prevalence of MMTV-like virus in BC is variable worldwide, ranging from 0 to 74% of cases [53].
Further, SV40 DNA sequences were detected in 22.9% of tumors and were not found in the corresponding non-tumor tissues. The positive rate was in line with the previous study conducted by Hachana et al. [28]. However, Martini et al. [54] concluded that the low prevalence of SV40 in samples from BC women and healthy women suggests no association between SV40 infection and BC. SV40 is likely a passenger not involved in the development of BC, in addition to possible endogenous contamination by SV40 sequences. Despite this, numerous epidemiological studies do not rule out a link between SV40 and cancer prevalence, since the virus can transform human cells.
Otherwise, the prevalence of viral genomes in tumors was analyzed for possible association with clinicopathological parameters. In our overall population, none of the four viruses was correlated with age. There was, however, a positive association between almost all viruses and histological grade, except for MMTV. Furthermore, our results demonstrated the importance of tumor stage for HPV and EBV. On the other hand, tumor size was only associated with EBV.
Interestingly, our results showed a trend toward a significant association between MMTV and histological type, as well as PRs and ERs. Indeed, MMTV was detected in 86.2% of invasive ductal carcinoma. Consistent with our study, Mazzanti et al. [55] reported the presence of an MMTV-like env sequence in 82% of ductal carcinomas in-situ. In contrast to our results, a study conducted in Morocco by Slaoui et al. [51], revealed no significant correlation between the presence of MMTV-like sequences and histological subtype and hormone receptors. In another study, Taneja et al. [22], similarly found that the presence of PR correlated significantly with MMTV-like positive BC, concluding that steroid hormones and MMTV may play a causal role in the development of BC.
Furthermore, our results showed a significant correlation between EBV and histological grade, tumor size, as well as PR and ER expression. In agreement with our results, Zhang et al. [50] found that EBV infection correlated significantly with tumor size and ER and PR (all P < 0.05). In contrast, they found no correlation with histological grade [50]. ER/PR receptors play a well-established role in the management and prognosis of BC [56].
Our findings analysis corroborates those of Kroupis et al. [57] and Kouloura et al. [58], who revealed a statistically significant relationship between HPV-positive BCs and PR-positive expression (P = 0.041) and histological grade (P = 0.005), respectively. However, they disagree with the outcomes of the research conducted by Elagali et al. [59].
Moreover, only 4 (5.71%) of the IBC samples in our investigation had coexistence of EBV, HPV, and MMTV infection, and there was no significant correlation between these findings and the clinicopathological characteristics. This result differs slightly from that of research conducted by Gupta et al. [60], who found triple co-infection in just 3% of samples of human triple-negative BC. The findings of Naushad et al. [4] also showed that 2.4% of samples were positive for all three viruses in Pakistani BC patients. Consistently, another study by Glenn et al. [2] in Australia, showed that the rate of co-infection of these three viruses in BC appears to be extremely low.
Moreover, available evidence clearly indicates that certain viruses play a role in the development of cancer. Above all, data on the maintenance of malignancy after loss of viral “presence” are enough to support the theory of viral cancer emergence. The hit-and-run theory will be put to the test by observing the results of long-term vaccination. According to this theory, viruses may play a triggering role at an early stage of carcinogenesis, such as cancer initiation (the “Hit”), and then the viral genome can be completely lost after the host cell has acquired many mutations and cancer has reached a developed stage (the “Run”). Alternately, viral infection at an advanced stage of cancer development could promote oncogenic features including growth, invasiveness, angiogenesis, and metastasis [61]. This theory explains the multi-stage manner in which virus-induced BC develops and progresses.
Furthermore, it has been demonstrated that HPV-cancer cells can only proliferate when the E6 and E7 oncogenes are expressed in cases of cervical, head, and neck cancer [62]. Other cancers such as BC and prostate cancer may be experiencing “hit-and-run” occurrences [63, 64]. BC development is initiated by E6 and E7 oncogenes, but as mutations are generated during carcinogenesis, the viral genes are eliminated since they are no longer needed [64]. In addition, Arbach et al. [61] reviewed several studies showing that many EBV-positive tumor cells do not contain EBV genomes, that the carcinogenic phenotype is unaffected by the loss of EBV episomes, and that BC exhibit genomic heterogeneity.
If the “hit-and-run” theory proves true, viruses could be responsible for more cancers than previously thought, and vaccination programs could therefore help prevent many cancers.
Although the prevalence of HPV, EBV, and MMTV co-infection is low, the synergistic carcinogenic impact of the different viruses in BC pathogenesis remains possible. We propose that the initiation and advancement of various malignancies, including BC, can be caused by interactions between the oncoproteins of HPVs (E6 and E7), and those of EBV (LMP1 and/or EBNA1) via EMT pathway, as well as with MMTV-like virus [60, 65, 66]. By promoting IFN signaling and APOBEC-mediated mutagenesis in BC, the MMTV-like virus contributed to the growth of tumors and the initiation and progression of BC [60].
Furthermore, it has been reported that a chronic inflammatory state brought on by the co-infection of another virus may enhance EBV, HPV, or MMTV-induced carcinogenesis. According to evidence on coinfection, virus-infected cells may provide a favorable environment for subsequent viral infection by producing inflammatory cytokines. These conclusions contribute to a better knowledge of the immune system’s limitations in order to create more effective vaccinations to prevent viral infections responsible for BC [4].
The findings of these investigations called for additional research using a large sample size and appropriate cell biologic tools, in order to clarify the likely causative roles of these viruses in BC as well as those of other viruses. Moreover, a potential limitation of our study is that the selected MMTV primers may not entirely capture all exogenous MMTV variants, which could lead to under-detection in some samples, and may require alternative primer strategies for future investigations.
The data obtained on single and multiple HPV, EBV, MMTV, and SV40 infections in IBC tissues consolidate the idea of a viral etiology of IBC, but do not imply a causal link between co-infection of the four viruses and the development of IBC. Further studies on the roles and the action mechanisms of these viruses are required to support the perspective of a viral etiology of BC.
Abbreviations
| BC: | breast cancer |
| EBV: | Epstein-Barr virus |
| ER: | estrogen receptor |
| HPV: | human papillomavirus |
| IBC: | invasive breast cancer |
| MMTV: | mouse mammary tumor virus |
| PCR: | polymerase chain reaction |
| PR: | progesterone receptor |
| SV40: | Simian virus 40 |
| Tag: | antigen T |
Declarations
Acknowledgments
Special acknowledgment for support of Faculty of Sciences and Techniques, Mohammedia, University Hassan II of Casablanca and Ministry of National Education, Vocational Training, Higher Education and Scientific Research in Morocco.
Author contributions
KAT: Conceptualization, Data curation, Methodology, Formal analysis, Software, Investigation, Project administration, Writing—original draft. AS: Writing—review & editing, Project administration. SAS: Visualization, Validation. KN: Investigation. MB: Resources. MME: Project administration, Supervision. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The study protocol was approved by the Ethics Committee for Biomedical Research of the Faculty of Medicine and Pharmacy of Casablanca, Morocco (Reference 3/2018 on 30.04.2018).
Consent to participate
Free and informed consent was obtained from all patients involved in this research and the confidentiality of their personal information was well-respected according to ethical rules.
Consent to publication
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed in the course of the present study are available from the corresponding author upon reasonable request.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.