Affiliation:
Department of Radiobiology and Molecular Genetics, “VINČA” Institute of Nuclear Sciences-National Institute of the Republic of Serbia, University of Belgrade, 522 Belgrade, Serbia
Email: jelenarad89@gmail.com
ORCID: https://orcid.org/0000-0002-3005-7943
Affiliation:
Department of Radiobiology and Molecular Genetics, “VINČA” Institute of Nuclear Sciences-National Institute of the Republic of Serbia, University of Belgrade, 522 Belgrade, Serbia
ORCID: https://orcid.org/0000-0001-6100-3887
Affiliation:
Department of Radiobiology and Molecular Genetics, “VINČA” Institute of Nuclear Sciences-National Institute of the Republic of Serbia, University of Belgrade, 522 Belgrade, Serbia
ORCID: https://orcid.org/0000-0002-4769-2652
Affiliation:
Department of Radiobiology and Molecular Genetics, “VINČA” Institute of Nuclear Sciences-National Institute of the Republic of Serbia, University of Belgrade, 522 Belgrade, Serbia
ORCID: https://orcid.org/0000-0002-0012-2636
Explor Med. 2021;2:544–555 DOI: https://doi.org/10.37349/emed.2021.00070
Received: October 27, 2021 Accepted: December 02, 2021 Published: December 31, 2021
Academic Editor: Akiko Mammoto, Medical College of Wisconsin, USA
The article belongs to the special issue Reactive Oxygen Species (ROS) in Pathophysiological Conditions
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) play a fundamental role in regulating endothelial function and vascular tone in the physiological conditions of a vascular system. However, oxidative stress has detrimental effects on human health, and numerous studies confirmed that high ROS/RNS production contributes to the initiation and progression of cardiovascular diseases. The antioxidant defense has an essential role in the homeostatic functioning of the vascular endothelial system. Endogenous antioxidative defense includes various molecules and enzymes such as superoxide dismutase, catalase, glutathione reductase, and glutathione peroxidase. Together all these antioxidative enzymes are essential for defense against harmful ROS features. ROS are mainly generated from redox-active compounds involved in the mitochondrial respiratory chain. Thus, targeting antioxidative enzymes and mitochondria oxidative balance may be a promising approach for vascular diseases occurrence and treatment. This review summarized the most recent research on the regulation of antioxidative enzymes in vascular diseases.
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity worldwide, including a wide array of disorders—cardiac muscle diseases and diseases of the vascular system supplying the heart, brain, and other vital organs [1]. There is significant evidence that vascular oxidative stress (OxS) is the leading cause of CVD [2, 3]. OxS is a harmful consequence of the imbalance between production and removal of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in situations where biological systems in cells and tissues cannot detoxify these reactive products [4–7]. ROS and RNS are small reactive ions and molecules that are derived from oxygen metabolism [8–10]. Small amounts of ROS and RNS are constantly produced and involved in defense mechanisms against microorganisms [11]. High doses of ROS/RNS cause oxidative modifications of major cell macromolecules (lipids, proteins, carbohydrates, and DNA), which can be further used as markers of OxS [12, 13]. ROS plays an essential role in regulating endothelial function and vascular tone [4] in promoting systemic inflammation, endothelial dysfunction, and vascular remodeling [2, 4]. Additionally, superoxide anion (O2−) interacts with nitric oxide (NO), producing highly toxic peroxynitrite (ONOO−) and therefore decreasing NO availability for smooth muscle relaxation function [14, 15]. All these processes lead to vascular system diseases such as atherosclerosis and arterial hypertension [1]. Cellular homeostasis is essential for preventing OxS, and it is finely tuned through the expression and action of antioxidant enzymes and non-enzymatic mechanisms [16]. Cells contribute to antioxidant defense in the vascular wall through numerous antioxidant enzymes such as superoxide dismutases [manganese superoxide dismutase (MnSOD), copper-zinc SOD (CuZnSOD), extracellular superoxide dismutase (EcSOD)], catalase (CAT), glutathione (GSH) peroxidase (GPx), thioredoxin peroxidase, and heme oxygenases [2]. The principal intracellular antioxidant is GSH, which can scavenge ROS and RNS, and additionally, GSH can also indirectly perform antioxidant function acting as a cofactor for various enzymes [17]. Regarding non-enzymatic antioxidants, the most potent ones are vitamin A, vitamin C, bilirubin, α-tocopherol (vitamin E), and β-carotene, which are present in blood [2, 11].
Data used for this review are obtained by searching the electronic database [PUBMED/MEDLINE 1988-October 2021]. The main data search terms were: reactive oxygen species, reactive nitrogen species, OxS, antioxidant enzymes, vascular diseases, antioxidant enzymes and vascular diseases, cardiovascular disease, metabolic syndrome, mitochondrial chain, nicotinamide adenine dinucleotide phosphate [NAD(P)H], and xanthine oxidases. Additionally, abstracts from national and international diabetes and cardiovascular-related meetings were searched.
In physiological conditions, enzymatic and non-enzymatic antioxidant systems maintain an equilibrium between the production and neutralization of ROS and RNS [11]. Antioxidant enzymes lower ROS and RNS levels or counteract downstream cellular effects of excessive oxidation. In addition, antioxidant enzymes detoxify ROS/RNS into less reactive species serving as an intermediate defense against ROS/RNS [18]. The primary antioxidant enzymes are SOD, CAT, GPx, and GSH reductase (GR) [12, 18]. Highly reactive radical O2− is converted by SOD to the less reactive radical hydrogen peroxide (H2O2), which can further be dissolved by CAT or GPx [18].
Superoxide-dismutase is a group of metalloenzymes that have a major antioxidant role in human health. In humans, there are three types of SODs: cytosolic CuZn (SOD1), mitochondrial Mn (SOD2), and extracellular CuZn (SOD3, EcSOD) [18, 19]. Phylogenetic analysis of SOD genes in vertebrates showed homology between SOD1 and SOD3 genes. However, the similarity with the SOD2 gene was shown to be minimal [20]. Moreover, SODs are presented as the first and the most major line of antioxidant defense against ROS, particularly O2− [19, 20]. The reaction implies binding O2− to an oxidized form of the enzyme (Fe3+, Cu2+, and Mn3+ respectively), which results in acquiring a proton and releasing molecular oxygen. Furthermore, second O2− and proton bind to the reduced form of the enzyme (Fe2+, Cu+, and Mn2+), which results in liberating H2O2 and returning of enzyme to its oxidized form [19, 21]. All three forms of SODs contain specific metals, which are essential for their function. SOD1 and SOD3 contain Cu and Zn ions in their catalytic center, while SOD2 contains Mn ions in their catalytic center [18]. SOD1 is a highly abundant enzyme that is ubiquitously expressed in eukaryotes, and it has been found in the cytoplasm, nuclear compartments, lysosomes, and mitochondrial compartments of mammalian cells [20]. Studies with SOD1 male and female knockout animals suggested that SOD1 is located in the mitochondrial intermembrane space, which is fundamental for motor axon maintenance. In contrast, mutations of the SOD1 gene that result in various single amino acids have been linked to familial amyotrophic lateral sclerosis [20, 22]. SOD2 is a tetramer enzyme located in mitochondria within the mitochondrial matrix, the main leading free radical production site from the electron transport chain [23]. Thus, SOD2 is a primary antioxidant enzyme in mitochondria essential for protecting respiring cells from oxidative damage [24]. Studies have shown that SOD2 knockout male mice die 2–3 weeks after birth due to cardiomyopathy and neurodegenerative diseases [20, 25]. Although there is substantial evidence supporting the idea of biochemical defects in the mitochondria in Parkinson’s and Alzheimer’s disease, there is little evidence to suggest the direct involvement of SOD2 in the clinical progression of the diseases [23]. Concerning SOD3, it contains a signal peptide that leads this enzyme to extracellular spaces, and it is probably located along the whole depth of the vascular wall [20, 26]. SOD3 is highly restricted to the specific cell types and tissue where it can exceed the activity of SOD1 and SOD2 [20, 26].
CAT exists as a tetramer enzyme that consists of four polypeptide chains with four ferriprotoporphyrin prosthetic groups per molecule [11]. CATs react with H2O2, breaking it down into molecular oxygen and water. Since one molecule of CAT hydrolyses over a million molecules of H2O2 per second, it is considered a highly effective enzyme [11, 27]. CATs are found in a wide range of aerobic and anaerobic organisms and are divided into three groups based on their structure and function [27, 28]. The first and the second groups are heme-containing enzymes called typical CAT and CAT peroxidases, whereby the third group contains Mn ions in their catalytic center and are called Mn CAT [28]. Numerous studies detected CAT gene mutations in patients with diabetes mellitus, hypertension, and vitiligo [29].
GPxs are enzymes that catalyze the reduction of H2O2 to water or hydroperoxides to corresponding alcohols using reduced GSH [30]. This reaction involves the formation of a disulfide bridge between two GSH molecules, creating a GSH disulfide, an oxidized version of GSH (H2O2 + 2GSH → oxidized glutathione (GS-SG) + 2H2O) [30, 31]. When the GSH molecule receives one electron from ROS, it becomes reactive, especially towards thiols, whereas the most abundant thiols in the cells are usually other GSH molecules, so dimer formation is favored [31]. Furthermore, recycling oxidized GSH back to its reduced form is catalyzed by enzyme GR, whereby this reaction requests the involvement of flavin adenine dinucleotide (FAD) and reduces NAD(P)H [31].
In mammalian tissues, there are four major GPx isozymes with selenocysteine in their active sites and two isozymes that are closely related to GPx3 [30, 32]. Their expression is tissue-dependent, whereas GPx1 is found in red cells, liver, lung, and kidney, GPx2 is found in gastrointestinal tracts, GPx3 is present in different organs such as kidney, lung, epididymis, vas deferens, placenta, seminal vesicle, heart and muscle and GPx4 which is widely distributed in various tissues [30]. GPx5 is found in the epididymis and lacks the inactive selenocysteine, while GPx6 is located in the olfactory epithelium [30]. Their subcellular locations are present in cytosol, nucleus, mitochondria and bound to membranes [30, 31]. Moreover, GR activity is also present in cytosol, nucleus, and mitochondria, and GR is found in the endoplasmic reticulum and the lysosomes [31].
ROS have physiological and pathological implications in cardiovascular tissues [33]. Low amounts of ROS generation are essential for cell functioning/signaling and, this process is called redox signaling [34, 35]. Also, subcellular ROS is significant for tissue adjustment to injury [36]. Various molecules participate in redox signaling, including hydroxyl radical (OH.), H2O2, transcription factors, NO, O2−, and all manifest protective actions in vascular physiology function [4, 33]. As far as cellular signaling, ROS alter cell signal pathway, gene expression, cellular proliferation, and protection versus infections [4]. In respect of vascular physiology, ROS may influence endothelium, cell differentiation and migration, vascular tone, and NO suppression [4]. However, in pathological states, ROS can induce oxidative disbalance of all cell components, along with proteins, lipids and nucleic acids [4, 37]. Thus, OxS is remarkably involved in heart failure and myocardial infarction development (Figure 1) [33].
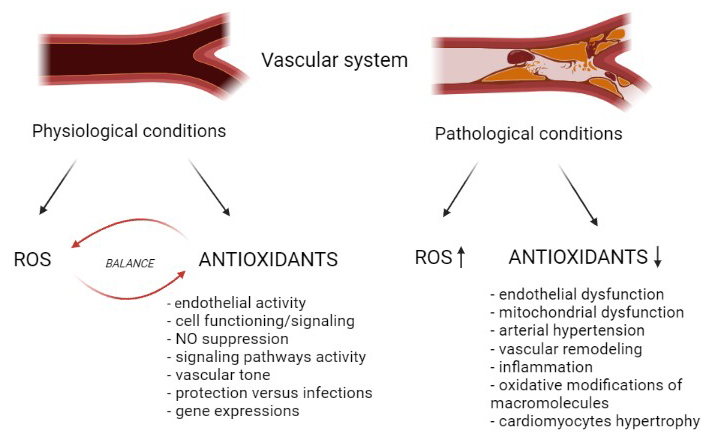
The impact of disbalance between ROS/RNS and antioxidant enzymes in OxS development in vascular diseases. It was created with Biorender.com
Chronically increased levels of ROS may participate in endothelial and mitochondrial dysfunction, atherosclerosis, hypertension development, and cardiomyocytes hypertrophy [33]. Enzymatically, O2− is mainly generated through the medium of NAD(P)H and xanthine oxidase (XO) [1]. NAD(P)H and XO oxidase are the primary molecules in the ROS pathology of vascular diseases [4]. Activated XO may attach to vascular cells and causes NO reduction and ONOO− escalation [38]. Further, increased XO activity may be essential for metabolic syndrome development since it generates O2− and reduces SOD activity, leading to endothelial dysfunction [39]. NAD(P)H oxidase is a pro-oxidant enzyme complex that generates H2O2 and O2− through NAD(P)H genes, which create the transmembrane proteins responsible for electron transport [40, 41]. Various NAD(P)H isoforms are expressed in human vascular smooth muscle cells (VSMCs) and endothelium, such as NOX1, NOX2, NOX4, and NOX5 [42]. This enzyme induces OxS which is associated with various pathologies, such as hypertension, coronary artery disease, atherosclerosis, diabetes mellitus, etc. [40, 43–45].
Targeting distinct agents which are responsible for OxS in vascular tissues seems to be a promising therapeutic approach [42]. According to a newly updated cohort study, the application of XO inhibitor is positively correlated with the decreased risk of vascular diseases in male and female human patients [46]. Further, using an in silico approach, some authors present new NAD(P)H inhibitors for hypertension treatment [47]. Hence, the management of NAD(P)H and XO oxidases and their inhibitors expressed in the VSMCs might be a potential target for vascular diseases medication that would inhibit the only pathological activity of NAD(P)H [40, 42, 48]. The endothelium is essential for vascular homeostasis and blood fluidity, and vascular tone balance [49]. In the endothelial cells, NO has a vital role in the proportion of vascular homeostasis. Since excessive ROS causes NO bioactivity reduction, endothelial dysfunction directly affects vascular blood vessels [49, 50]. Decreased NO generation occurs due to reaction with O2−, produced from NAD(P)H and XO oxidase, and consequently, ONOO− originates, leading to apoptosis of endothelial cells [49, 51, 52].
However, some authors point out some positive aspects of small ROS amount in endothelial cell generation and metabolism [53]. Endothelial cells can generate ATP in anaerobic conditions and store oxygen, and this paradox brings decreased ROS levels and rapid ATP generation [53]. Nonenzymatically, O2− is generated when oxygen reacts with redox-active compounds [1], especially from the mitochondrial respiratory chain (dominant source of intracellular ROS) [33]. Mitochondria are especially abundant in cardiac tissue [49]. Thus, increased ROS generation in the course of mitochondrial dysfunction is an essential factor for the occurrence and development of atherosclerosis, high blood pressure, heart failure, and ischemia-reperfusion injury [54]. For instance, one study with a male murine model of myocardial infarction induced by coronary ligation, OH., and lipide peroxide values in the mitochondria was elevated [55]. Besides, ROS generated in mitochondria is related to vascular complications in diabetes, especially cardiomyopathy [54, 56]. According to new data, in male Wistar rats with induced diabetes, a significant increase of ROS and decrease of CAT activity has been recorded in the heart muscle [57]. In the same study, mitochondrial complexes I, II, III, and IV were reduced, and SOD2 and GSH/glutathione disulfide (GSSG) ratio values decreased in heart mitochondria in diabetic rats [57]. Regarding mitochondrial ROS participation in vascular disease pathogenesis, targeting mitochondria and their oxidative balance may be a promising approach for vascular complications medication.
A protective system for free radical excess removal is generated during the evaluation, whereas all antioxidants represent an antioxidant defensive system [58]. Antioxidants are divided into two groups: enzymes, the primary line of antioxidative defense (SOD, CAT, GPx, GR, GSH) and non-enzymes, the secondary line of antioxidative defense (vitamins E and C, albumin, thiols, β-carotenes, etc.) [59–62].
One of the most important antioxidant enzymes is SOD, which catalyzes O2− into oxygen and H2O2 [63, 64]. SOD2 stands up against mitochondrial ROS and can minimalize vascular calcification among VSMC [65, 66]. In male and female patients with idiopathic pulmonary arterial hypertension, the expressions of all three SOD isoforms were reduced compared to the healthy patients [67]. Nevertheless, gene rs7655372 locus polymorphism of SOD3 in male and female patients is a risk factor for ischemic stroke [68]. The association between SODs activities and vascular diseases is imperative for developing a new diagnostic biomarker and therapy strategy.
Regulation of CAT implicates OxS-associated pathways and diverse transcription factors, for instance, nuclear factor Y (NF-Y), peroxisome proliferator-activated receptor δ (PPAR δ), specificity protein 1 (Sp1), etc. [69–72]. CAT protective role versus ROS is established, and according to some authors, reduction of CAT activity enhances abdominal aortic dilatation appearance [73]. Further, cardiac CAT activation by adipokine apelin during hypertrophic remodeling manifests protective features versus ROS in male C57BL6/J mice and cultures of cardiac myocytes [74]. Similarly, in experimental male rats, CAT reactivation by curcumin in the heart and aorta displays protective properties against lipopolysaccharide [75]. In addition, results from Dai et al. [76] study showed that CAT upregulation in mitochondria protects male and female mice from vascular aging.
Another intracellular antioxidant enzyme, GPx1, transforms H2O2 to H2O and lipid peroxides to alcohols and plays a significant role in ROS prevention [77]. In apolipoprotein E and GPx1 deficiency (ApoE−/−GPx1−/−) female mice, atherosclerosis and plaque lesions were more expressed than those without GPx1−/− [78]. The same authors declared that GPx1−/− causes ROS elevation in the aorta of the animals [78]. In another animal study with male ApoE−/− mice overexpressing GPx4, atherosclerosis progression and lipid peroxidation were suppressed in aorta endothelial cells [79]. According to new data, GPx1 protein expression was significantly reduced in male mice aortic tissue with induced diabetes [80]. GR also manifests a protective feature against OxS, and its overexpression in heart tissue of a Klotho-hypomorphic (antiaging gene) deficient male mice resulted in heart failure and apoptotic prevention [81]. GSH has a significant part in the antioxidant defense system and cell homeostasis and metabolism. Nevertheless, GSH deficiency has an essential part of aging and cardiovascular pathology [82].
Although data from studies with experimental animal models advocate the protective role of antioxidants versus vascular disorders [83–86], data from clinical trials are not conclusive [56, 87]. For example, vitamin A has been rated as a beneficial supplement that may reduce OxS in diabetic individuals with ischemic heart disease [88]. Melatonin supplementation may reduce myocardial ischemic-reperfusion injury in male and female patients undergoing coronary artery bypass [89]. Further, vitamins E, A, and C decrease blood pressure in male patients with hypertension [90]. Consistent with these statements, a new meta-analysis that included 11 cross-sectional studies and 7 case-control studies with both gender individuals concluded that vitamin C positively influences blood pressure and endothelial function [91]. Still, long-term trials with an extensive number of participants are necessary for clarification of vitamin C benefit role in cardiac complications [92, 93]. A large randomization study indicated that vitamin E supplementation at high doses might even elevate the risk for coronary artery disease development [94]. Indeed, treatment with vitamin E for an extended time did not affect vascular events in male and female individuals with diabetes or other cardiovascular comorbidities [95]. Micronutrient selenium, which is considered a potent antioxidant, failed to reduce chronic chagasic cardiomyopathy in 66 male and female patients, according to a randomized, placebo-controlled, double-blinded clinical trial [96]. Ye et al. [97] analyzed data from 15 trials and 188,209 participants and revealed that vitamins C and E, together with β carotene, had no positive impact on cardiovascular complications. It cannot be denied that reducing OxS via antioxidants is important; however, up to now, the results of clinical studies have been predominantly pessimistic [98]. We assume that there are several reasons for this attitude. Most of the clinical trials regarding vascular comorbidities have been examined a single antioxidant, so antioxidant combinations and their molecular basis might be discussed in future investigations [3]. Secondly, OxS should be identified through enzymes activity rather than produced molecules [98].
The impact of OxS is detrimental to human health, and numerous studies confirmed that high ROS production contributes to the initiation and progression of CVD [5, 99, 100]. Thus, the antioxidant defense has an essential role in the homeostatic functioning of the vascular endothelial system, whereby antioxidant enzymes represent the primary line of antioxidant protection [4, 59]. Numerous studies have proven the association between reduced expression and activity of antioxidant enzymes and CVD development [68, 73, 80]. Hence, additional research on how ROS is utilized in the cardiovascular system and consequently impacts the regulation of antioxidant enzymes is needed to develop new diagnostic biomarkers and therapeutic strategies.
CAT: catalase
Cu: copper
CVD: cardiovascular disease
GPx: glutathione peroxidase
GR: glutathione reductase
GSH: glutathione
H2O2: hydrogen peroxide
Mn: manganese
NAD(P)H: nicotinamide adenine dinucleotide phosphate
NO: nitric oxide
O2−: superoxide anion
ONOO−: peroxynitrite
OxS: oxidative stress
RNS: reactive nitrogen species
ROS: reactive oxygen species
SOD: superoxide dismutase
XO: xanthine oxidase
Zn: zinc
JR and KB wrote the manuscript and contributed conception. ERI and MO designed, wrote, and supervised the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was funded by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Contract No#451-03-9/2021-14/200017). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Yuka Ikeda ... Satoru Matsuda
Ranjeet Singh, Partha Pratim Manna
Anastasija Panic ... Esma R. Isenovic
Tao Wang, Haiyan Xu
Juanjuan Fei ... Jun Ren
Alberto Rubio-Casillas ... Raied Badierah
Largee Biswas ... Anita Kamra Verma
