Abstract
Aim:
The present study investigated the appearance and severity of atrial fibrillation (AF) and heart failure (HF) in 8,702 hypertensive patients with left ventricular hypertrophy (LVH) receiving antihypertensive treatment in a prospective trial.
Methods:
Patients who had a history of AF or HF were not included, and the participants had sinus rhythm when they were randomly allocated to blinded study medication. Endpoints were adjudicated.
Results:
Incident AF occurred in 679 patients (7.8%) and HF in 246 patients (2.8%) during 4.7 ± 1.1 years mean follow-up. Incident AF was associated with a > 4-fold increased risk of developing subsequent HF [hazards ratios (HRs) = 4.7; 95% confidence intervals (CIs), 3.1–7.0; P < 0.001] in multivariable Cox analyses adjusting for age, sex, race, randomized treatment, standard cardiovascular risk factors and incident myocardial infarction. The development of HF as a time-dependent variable was associated with a multivariable-adjusted 3-fold increase of the primary study endpoint (HRs = 3.11; 95% CIs, 1.52–6.39; P < 0.001) which was a composite of myocardial infarction, stroke or cardiovascular death. Incident HF was associated with a > 3-fold increased risk of developing subsequent AF (HRs = 3.3; 95% CIs, 2.3–4.9; P < 0.001). This development of AF was associated with a > 2-fold increase of the composite primary study endpoint in multivariable Cox analysis (HRs = 2.26; 95% CIs, 1.09–4.67; P = 0.028).
Conclusions:
Incident atrial fibrillation and heart failure are associated with increased risk of the other in treated hypertensive patients with left ventricular hypertrophy. Such high-risk hypertensive patients who subsequently develop both atrial fibrillation and heart failure have particular high risk of composite myocardial infarction, stroke or cardiovascular death (ClinicalTrials.gov identifier: NCT00338260).
Keywords
Atrial fibrillation, blood pressure, hypertension, heart failure, the LIFE studyIntroduction
Hypertension may cause atrial fibrillation (AF) and heart failure (HF), partly due to higher intraventricular pressure causing left ventricular hypertrophy (LVH) and left atrial dilatation. Heart failure due to hypertension may be the leading cause of HF with preserved ejection fraction due to concomitant LVH and depressed left ventricular myocardial function [1]. Besides hypertension, both AF and HF increase the incidence of cardiovascular events. Thus, AF and HF are responsible for substantial increases in morbidity and mortality as well as financial cost [2–4]. These two conditions often co-exist in part because they share antecedent risk factors including hypertension, but also because one may directly predispose to the other [5]. Individually, AF and HF increase the risk of stroke and cardiovascular death, and the synergistic combination of AF and HF creates a pro-thrombotic state that results in greater stroke morbidity and mortality than the mere presence of either condition separately.
In the Framingham Heart Study, both prevalent and incident AF predicted new-onset HF and vice versa [6] and participants with AF or HF who subsequently developed the other condition had increased risk of adverse outcome including mortality [5, 6]. However, other studies have failed to demonstrate an independent association of AF with mortality in HF patients [7, 8]. It remains unclear whether incident AF predicts new-onset HF or vice versa particularly in high-risk patients with hypertension and LVH. Furthermore, whether onset of AF after HF is associated with higher risk of cardiovascular events than vice versa, in these high-risk hypertensive patients remains largely unknown. The present study therefore aimed to investigate these questions in a cohort of hypertensive patients with electrocardiographic LVH.
Materials and methods
Materials
The Losartan Intervention For Endpoint reduction in hypertension (LIFE) study enrolled patients aged 55–80 years with previously treated or untreated moderate to severe hypertension and LVH by electrocardiogram in a prospective, double-blind, multicenter, randomized study to determine whether greater reduction in cardiovascular events is achieved by losartan-based rather than atenolol-based treatment [9, 10]. Patients with secondary hypertension, left ventricular ejection fraction ≤ 40% or a history of renal or hepatic disorders with severe impairment of function (serum creatinine > 160 μmol/L) were excluded. History of AF or AF in baseline electrocardiogram (ECG) as well as suspected previous HF was additional exclusion criteria for the present study.
Study electrocardiograms were taken at baseline, at 6-months and at yearly follow-up intervals until study termination or patient death and were interpreted as previously reported in detail [11]. Cornell product > 2,440 mV × ms or Sokolow–Lyon voltage > 38 mV were used to identify LVH [10–13]. New-onset AF was identified from protocol-mandated in-study electrocardiograms undergoing Minnesota coding at the Electrocardiographic Core Laboratory and/or by adverse event reports of AF by the investigators [14]. Each case was reviewed and verified by the Endpoint Committee who was masked for patients’ history and clinical findings when classifying possible morbid events [9, 14]. The diagnosis of incident HF was based on clinical and diagnostic findings modified from Framingham criteria [15]. The primary endpoint in the LIFE study, also adjudicated by the Endpoint Committee, was a composite of acute myocardial infarction, cerebral stroke and cardiovascular death. The study was endpoint driven and needed 1,040 primary endpoints to achieve adequate statistical power [10].
Statistical analyses
The temporal relationship of incident AF to incident HF was assessed in 8,702 patients with no history of AF or HF, and in sinus rhythm at baseline. In addition, this study tested whether the development of HF in AF patients was associated with higher risk of subsequent cardiovascular events (a composite endpoint of the first event of myocardial infarction, stroke, and cardiovascular death) compared to the development of AF in HF patients. We used time-varying multivariable Cox models to estimate hazards ratios (HRs) and confidence intervals (CIs). The multivariable analyses were adjusted for age, gender, diabetes and study treatment, and hemoglobin, time-varying systolic and diastolic blood pressure, time-varying Cornell electrocardiographic LVH, time-varying QRS-duration, time-varying heart rate, time-varying myocardial infarction, low-density lipoprotein (LDL)- and high-density lipoprotein (HDL)-cholesterol, total cholesterol, creatinine, glucose, albumin–creatinine ratio, and race. Proportional hazard assumptions and lack of interactions were tested for each model and confirmed unless otherwise reported. Differences in risk of incident AF in respect to incident HF and vice versa are presented in forest plots. SAS statistical software package version 9.4 for PC (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses. Two-tailed P < 0.05 was regarded as statistically significant.
Results
A total of 362 patients with a history of AF (n = 342) and/or AF on their LIFE baseline electrocardiograms (n = 135) were excluded as were 129 patients suspected of previous HF, leaving 8,702 patients without AF or HF by history or baseline electrocardiogram in the present study.
Incidence rates for developing the comorbid event
During 4.7 ± 1.1 years mean follow-up, 837 participants developed AF, HF, or both. Baseline characteristics of patients according to their initial event are shown in Table 1. The mean follow-up was 2.4 years (1,500 persons–years) after the development of incident AF and 2.7 years (516 persons–years) after the development of incident HF. The composite endpoint occurred in 958 (11%) patients. Eighty-eight patients developed both AF and HF. Of these, 34 had AF first, 33 had HF first, and 21 had both disorders diagnosed on the same day (Figure 1). The majority of patients with incident AF (n = 591) did not develop HF (Figure 1). Similarly, 158 patients developed HF but without AF (Figure 1).
Baseline characteristics in groups of patients by initial event, atrial fibrillation, heart failure or both
| Variable | Incident AF (n = 625) | Incident HF (n = 191) | AF and HF on same day (n = 21) |
|---|---|---|---|
| Age (years) | 69.8 ± 6.5 | 70.4 ± 6.4 | 70.3 ± 7.8 |
| Women, n (%) | 310 (49.6) | 89 (46.6) | 10 (47.6) |
| Systolic blood pressure (mmHg) | 152 ± 21 | 154 ± 23 | 138 ± 22 |
| Diastolic blood pressure (mmHg) | 84 ± 11 | 85 ± 13 | 79 ± 13 |
| Heart rate (beats/min) | 72 ± 14 | 74 ± 13 | 75 ± 20 |
| Body mass index (kg/m2) | 27.8 ± 4.8 | 28.4 ± 5.2 | 30.3 ± 7.2 |
| Serum creatinine (μmol/L) | 87 ± 22 | 95 ± 24 | 82.7 ± 16.0 |
| Total cholesterol (mmol/L) | 6.0 ± 1.1 | 5.9 ± 1.2 | 5.6 ± 1.3 |
| HDL cholesterol (mmol/L) | 1.5 ± 0.4 | 1.4 ± 0.4 | 1.4 ± 0.5 |
| Plasma glucose (mmol/L) | 6.0 ± 2.1 | 6.8 ± 3.6 | 6.1 ± 2.0 |
| Cornell voltage–duration product (mV∙ms) | 2,781 ± 1,111 | 3,460 ± 1,708 | 2,964 ± 1,402 |
| Sokolow–Lyon voltage (mV) | 31.4 ± 11.6 | 32.7 ± 11.1 | 35.2 ± 12.6 |
| QRS-duration (ms) | 103 ± 20 | 115 ± 26 | 113 ± 22 |
| Prior myocardial infarction, n (%) | 22 (3.5) | 16 (8.4) | 0 |
| Diabetes, n (%) | 83 (13) | 51 (27) | 4 (19) |
| Black race, n (%) | 20 (3) | 21 (11) | 3 (14) |
| Randomized to atenolol, n (%) | 280 (45) | 94 (49) | 12 (57) |
Without concurrent or prior heart failure; without concurrent or prior atrial fibrillation; measured at day of event
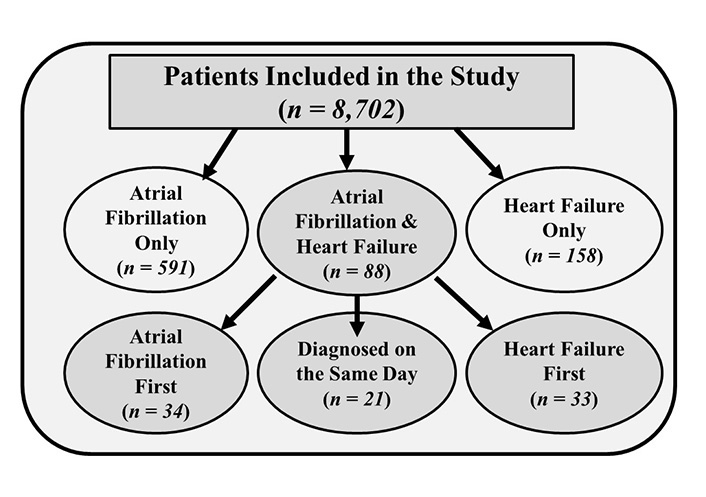
Timing and distribution of patients with atrial fibrillation, heart failure, or both
Impact of developing atrial fibrillation on heart failure and vice versa
In multivariable Cox analyses, incident AF as a time-varying covariate was associated with a > 4-fold increased risk of developing subsequent HF (HRs = 4.7; 95% CIs, 3.1–7.0; P < 0.001, Figure 2) after adjustment for covariates. In a parallel multivariable analysis, time-varying new-onset HF was associated with a > 3-fold increased risk of subsequent AF (HRs = 3.3; 95% CIs, 2.3–4.9; P < 0.001, Figure 2).
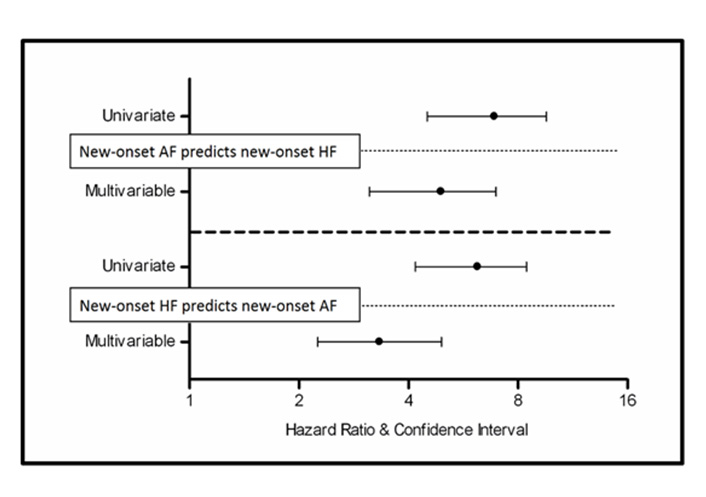
Incident (new-onset) atrial fibrillation predicts incident (new-onset) heart failure and vice versa in treated hypertensive patients. The upper part of this figure shows that incident (new-onset) atrial fibrillation was associated with a > 4-fold increased risk of developing subsequent heart failure in both univariate and multivariate analysis; the lower part of this figure shows that incident (new-onset) heart failure was associated with a > 3-fold increased risk of developing subsequent atrial fibrillation in both univariate and multivariate analysis
Impact of developing the comorbid condition on the composite endpoint
Multivariable Cox models were used to evaluate the impact of AF on the composite endpoint in HF patients, restricting our analyses to those who were free of AF at the time of HF diagnosis. The development of subsequent AF (time-dependent variable) was associated with a > 2-fold increase of the composite cardiovascular endpoint in multivariable Cox analysis (HRs = 2.26; 95% CIs, 1.09–4.67; P = 0.028).
Similarly, we examined the impact of HF on the composite endpoint in AF patients, restricting our analyses to those patients who were free of HF at the time of AF diagnosis. The subsequent development of HF (time-dependent variable) was associated with a multivariable-adjusted 3-fold increase of the composite cardiovascular endpoint (HR = 3.11; 95% CIs, 1.52–6.39; P < 0.001).
Discussion
Our study shows that incident AF and incident HF are each associated with an increased risk of the other in treated hypertensive patients with LVH, although the risk of HF seemed larger in patients developing AF than vice versa. Furthermore, the risk of composite cardiovascular events including myocardial infarction, cardiovascular mortality and stroke was greater in patients developing AF before HF than vice versa in these hypertensive patients.
Hypertension increases the risk of AF and the relative risk of developing AF in hypertensive patients has been estimated to be 1.4–2.1 [16]. Consistent with this, our group has previously demonstrated that achievement of a systolic blood pressure of ≤ 130 mm Hg is associated with a decreased risk of incident AF, independent of standard AF risk factors, compared with higher levels of systolic blood pressure [17]. This benefit of blood pressure control during anti-hypertensive treatment may at least partly be explained by regression of LVH [14] and/or reduced atrial dimension [18].
In parallel, hypertension is a well-known precursor to development and progression of HF. After adjusting for age, the Framingham Heart Study showed that the incidence of HF was two- to three-fold greater in hypertensive compared to normotensive study participants [19]. This association may be mediated by several conditions including myocardial infarction, sympathetic nervous system over-activity and LVH. Supporting the latter possibility, the LIFE study revealed, that regression of LVH was associated with less incident HF in hypertensive patients [20].
In the present study, incident AF inserted as a time-varying covariate was associated with a > 4-fold increased risk of developing subsequent HF independent of established risk factors including in-study blood pressure and LVH, as well as in-study occurrence of myocardial infarction. In a parallel multivariable analysis, incident HF inserted as a time-varying covariate was independently associated with a > 3-fold increased risk of subsequent AF. The strong association of incident AF with increased risk of subsequent HF may be explained by the higher ventricular rate and stress in AF potentially leading to HF by causing tachycardia-induced cardiomyopathy.
A plausible contributor to incident AF in HF patients is propagation of elevated left ventricular diastolic pressure in HF to the left atrium through the mitral valve, stretching the left atrium and thereby stimulating increased atrial and pulmonary vein automaticity as well as impaired atrial conduction. The less strong association of incident AF in HF patients shows that HF in less degree leads to AF than vice versa. A possible explanation could be that more chronically underlying conditions such as hypertension, aging, atrial fibrosis, aortic stenosis, myocardial infarction or left ventricular overload might play a more significant role in the development of AF in patients with HF than vice versa. In addition, potential pathways by which HF leads to AF also include tethering of the mitral valve causing mitral regurgitation. The clinical phenotypes of HF with and without mitral regurgitation are associated with very different rates of cardiovascular morbidity and mortality [21]. In the Framingham study, there were similar percentages of participants who developed AF in HF participants as vice versa. However, the Framingham study participants were followed for more than a decade, which is more than twice as long as the patients in the LIFE study, leaving more time for the more chronic AF to develop in the HF patients [5, 6].
It is controversial whether AF per se increases the risk of morbidity and mortality. The LIFE study, which included hypertensive patients with LVH, showed that incident AF was independently associated with > 3-fold increased risk of sudden cardiac death, 3-fold increase of fatal and non-fatal stroke, and 5-fold increased risk of hospitalization for HF [22, 23]. Subsequently, the AF associated risk was confirmed in a Danish national study including 89,702 patients with myocardial infarction, showing that incident AF was associated with a 2-fold increase in all-cause mortality, cardiovascular death, stroke, and re-infarction.
However, taken together it may be more of an academic discussion regarding the importance of what comes first of HF or AF. The pathophysiological pathway may be different but from a clinical point of view it does not matter very much whether treated hypertensive patients with LVH develop AF before HF of visa versa. It is important to realize that in hypertensive patients with LVH these two serious complications are frequently related. Both endpoints are important complications to prevent and we cannot differentiate whether one type of preventive treatment would be better than another type of preventive treatment.
In a community-based cohort, the Framingham Heart Study demonstrated that the combination of AF and HF portend greater risk of overall mortality than either condition alone [5, 6]. However, the Vasodilator Therapy in Congestive Heart Failure trial (V–HeFT) failed to show higher risk of all-cause mortality or sudden death associated with pre-existing AF in patients with mild to moderate HF [24]. Rhythm control has not proven to prevent poor outcome compared to rate control. In the Atrial Fibrillation and Congestive Heart Failure trial (AF–CHF) including 1,376 patients with AF and HF with reduced ejection fraction (“systolic” HF), amiodarone had no preventive effect on mortality in the rhythm group compared to the rate group [25]. However, most of these patients developed AF after HF and not the other way around. Therefore, it is possible that other more chronic comorbidities contributed more significantly to the prognosis in these patients than AF per se.
In the present study, incident AF in HF patients was associated with a > 2-fold increase of the composite endpoint of myocardial infarction, stroke and cardiovascular death in patients with HF independent of the usual predictors of AF. However, incident AF in HF patients was not associated with all-cause mortality. Conversely, incident HF in patients diagnosed with AF was independently associated with a multivariable-adjusted 3-fold increase of the composite endpoint and was significantly associated with all-cause mortality. This suggests that HF development in AF patients has more serious prognostic impact than vice versa. A reason for this could be that adaptation to HF is impaired in AF patients, with loss of normal ventricular rate control and of the booster pump effect of atrial contraction. Conversely, AF development in HF patients might reflect slow progression of HF that over several years leads to atrial fibrosis, atrial enlargement and increasing sympathetic activity resulting in AF [26]. In this case, the heart has more time to adapt to the association. However, in total and in summary, these differences in outcomes following first incident AF or HF may be more of an academic than a clinical issue. The strong results and conclusions of this LIFE sub-study are that new AF, new HF, or both, are statistically and clinically associated to an increased risk of developing the composite endpoint.
Wang et al. [5] demonstrated that HF patients who developed AF suffered higher mortality than HF patients who maintained sinus rhythm. Furthermore, in accordance with our results, they showed that AF patients who developed HF suffered even greater mortality. Interestingly, they also showed that among HF patients, neither prior nor concomitant AF was associated with all-cause mortality, compared to HF patients without AF. This finding confirms our finding of incident AF as a lower-risk condition in HF patients compared to incident HF in AF patients. Milani et al. [27] showed that when HF develops in a population with LVH, the patients are converting to low ejection fraction and possible poor prognosis from this point of view.
Diabetes mellitus type-2 is a risk factor for AF and HF as well. Some of us investigated the influence of preexisting and incident diabetes mellitus on developing incident AF and HF in the Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) Trial population of high-risk hypertensive patients [28]. Five thousand two hundred and fifty patients of the 15,245 participants in the VALUE trial had diabetes mellitus at baseline and 1,298 of the initially nondiabetic patients developed diabetes mellitus during the average 4.2-year follow-up. Five hundred and fifty-one patients developed AF during the trial. Patients with incident diabetes had a significantly higher event rate of incident AF compared with patients without diabetes and there was also a trend towards more incident AF in patients with diabetes at baseline. Patients with incident diabetes mellitus concomitant with incident AF had a > 3-fold risk for incident HF compared with patients with incident diabetes mellitus without AF. We concluded [28] that hypertensive patients in the VALUE trial who developed diabetes mellitus during the trial had more incident AF than did patients without diabetes mellitus, and that incident AF explained some of their concomitantly high risk of HF development.
Diabetes mellitus type-2 is increasingly common in the type of hypertensive patients enrolled in randomized outcomes studies like LIFE and VALUE. Our data [28] suggest that the pathophysiological mechanism is interrelated when diabetes mellitus-2 develops and the hypertensive heart disease is simultaneously worsening. Another issue is obesity as the population today is much more obese and could now have a higher probability of both HF and AF [29, 30].
Heart failure was a pre-specified secondary endpoint adjudicated by the endpoint committee according to Framingham criteria. There was no difference between losartan and atenolol in preventing HF, though a small imbalance made us include randomized treatment in our multivariate analysis in the present study. Thus, we feel quite strongly that randomized treatment did not influence our present findings except for perhaps a general prevention of developing HF. The question of additional diuretic treatment is as well interesting [31]; however, there was no difference between the two treatment arms whatsoever regarding additional treatment with diuretics [32].
Incident cases of HF were adjudicated according to Framingham criteria. Thus we feel confident that patients truly had HF—even without systematic taking of a biomarker such as N-terminal (NT)-prohormone brain natriuretic peptide (BNP) which has proven its position as diagnostic criterion in later research and most likely will become a key criterion in future guidelines. However, we cannot in LIFE detail whether patients developed HF with preserved ejection fraction (HFpEF) or reduced ejection fraction (HFrEF) since echocardiography or other method for EF determination was not a mandatory part of the diagnostic Framingham criteria.
In conclusion, high-risk hypertensive patients with AF or HF who subsequently develop the other condition have very high cardiovascular risk. Therefore, prevention and optimal management of the joint occurrence of AF and HF in patients with hypertension is important. Furthermore, the development of AF before HF seems to reflect a more severe condition that vice versa.
Limitations of this study
Our LIFE sub-study is a post-hoc analysis of a randomized controlled trial. Furthermore, the present sub-study was done in a population of patients with moderate to severe hypertension and electrocardiographic left ventricular hypertrophy; and without atrial fibrillation, heart failure or renal or hepatic disorders with severe impairment; and thereby may not be directly applicable to patients with isolated hypertension or patients with mild hypertension or severe impairment of renal or hepatic function.
One hundred and fifty eight patients developed heart failure without atrial fibrillation. Our study included 679 patients with apparent atrial fibrillation diagnosed by electrocardiogram obtained at 6-months and at yearly follow-up intervals until study termination or patient death. This type of detection may be the tip of the iceberg since a substantial proportion of patients may have had subclinical paroxysmal atrial fibrillation and remained undetected. Devices detected atrial fibrillation are tools these days in order to resolve these gaps and thus future research may possibly expand the role of atrial fibrillation in explaining stroke and heart failure in high-risk hypertension.
Abbreviations
| AF: | atrial fibrillation |
| CIs: | confidence intervals |
| HF: | heart failure |
| HRs: | hazards ratios |
| LIFE: | Losartan Intervention For Endpoint reduction in hypertension |
| LVH: | left ventricular hypertrophy |
| VALUE: | Valsartan Antihypertensive Long-Term Use Evaluation |
Declarations
Acknowledgments
The authors thank the 945 clinical centers who participated in the LIFE study.
Author contributions
CNB, SEK, KW, RBD and PMO contributed to the conception and design of this LIFE sub-study. CNB and PMO performed the statistical analyses. CNB wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflicts of interest
Lars Køber has received speakers’ honorarium from Novo, Novartis, AstraZeneca and Boehringer. Sverre E. Kjeldsen has received ad-hoc lecture honoraria within the past 3 years from Getz Pharma, Merck Healthcare KGaA, Sanofi-Aventis and Vector-Intas. The other authors declare that they have no conflicts of interest.
Ethical approval
Ethical committees for all participating 945 clinical centers approved the LIFE study. This manuscript complies with the Declaration of Helsinki.
Consent to participate
Written informed consent to participate in the study was obtained from all participants.
Consent to publication
Not applicable.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author (PMO) upon dire need.
Funding
Merck & Co., Inc. supported the LIFE study with an unrestricted grant from 1993. Merck had a non-voting member of the Steering Committee, which designed the study and wrote the protocol. Merck did monitoring, and accumulated data in a Merck database until study end in 2002. Merck provided the study steering committee with free access to blinded data until 2002 and then un-blinded data after the database was cleared for queries and frozen. The steering committee including a committee statistician validated independently the main outcomes. The steering committee has always been free to analyze and interpret the data, make decisions to publish and write the papers. Merck has not had any formal role after 2002 except for sporadically providing expert statistical assistance.
Copyright
© The Author(s) 2022.