Affiliation:
1Department of Internal Medicine 3 – Rheumatology and Immunology, Friedrich-Alexander University Erlangen-Nürnberg and Universitätsklinikum Erlangen, 91054 Erlangen, Germany
2Deutsches Zentrum für Immuntherapie, Friedrich-Alexander University Erlangen-Nürnberg and Universitätsklinikum Erlangen, 91054 Erlangen, Germany
3University Hospital Würzburg, Department of Internal Medicine 2, Rheumatology/Clinical Immunology, 97080 Würzburg, Germany
ORCID: https://orcid.org/0000-0001-5762-9182
Affiliation:
4Thermo Fisher Scientific, Product Innovation, Research and Development, 79111 Freiburg, Germany
ORCID: https://orcid.org/0000-0002-2516-5261
Affiliation:
1Department of Internal Medicine 3 – Rheumatology and Immunology, Friedrich-Alexander University Erlangen-Nürnberg and Universitätsklinikum Erlangen, 91054 Erlangen, Germany
2Deutsches Zentrum für Immuntherapie, Friedrich-Alexander University Erlangen-Nürnberg and Universitätsklinikum Erlangen, 91054 Erlangen, Germany
5Université Grenoble Alpes, AGEIS, 38000 Grenoble, France
Email: johannes.knitza@uk-erlangen.de
ORCID: https://orcid.org/0000-0001-9695-0657
Explor Musculoskeletal Dis. 2023;1:64–67 DOI: https://doi.org/10.37349/emd.2023.00010
Received: April 28, 2023 Accepted: June 15, 2023 Published: June 30, 2023
Academic Editor: Fernando Pérez-Ruiz, Basque Country University, Spain
The article belongs to the special issue Digital health technologies in rheumatology: emerging evidence and innovation
Dear Editor,
One of our recent studies highlighted the great potential of capillary self-sampling as a cornerstone for future rheumatology care [1]. In addition, the feasibility of capillary self-sampling in rheumatic patients for analysis of inflammatory markers [2], auto-antibodies [2, 3], and coronavirus disease (COVID) antibodies was demonstrated by us [4]. Human leucocyte antigen-B27 (HLA-B27) determination is still an important diagnostic component in the diagnosis of axial spondylarthritis (axSpA) as it is present in the majority of axSpA patients. Given the long diagnostic delay in axSpA [5, 6], capillary HLA-B27 determination, in particular, may be of interest as it may help to screen patients with inflammatory back pain and suspected axSpA independently of on-site visits. This prospective study investigated the accuracy, feasibility, and patient acceptance of unsupervised capillary blood self-collection for HLA-B27 analysis in a patient cohort with inflammatory back pain and suspected axSpA.
The study was approved by the institutional review board of the Medical Faculty of the University of Erlangen-Nürnberg (21-357-B) and conducted in compliance with the Declaration of Helsinki. A number of 36 newly referred patients with suspected axSpA were included and received a conformité européenne (CE)-certified upper-arm device (TAP II, YourBio Health, Medford, USA, Figure 1A) and a heat-pack to perform unsupervised capillary blood self-collection at home. Patients only had access to the standard instructions, including a leaflet with written instructions and a link to a video. After increasing blood circulation at the chosen collection site by applying the heat pack for 1 min, patients attached the device to the upper arm with an adhesive, and small microneedles get activated by pressing a button. Upon skin puncture, the device applies a vacuum and collects blood in an attached tube. The blood sealed in the collection tube was shipped by patients to Thermo Fisher Scientific (Freiburg, Germany) for analysis using regular postage. Upon arrival in the laboratory, 50 µL of whole blood was stored at –20°C in RNase-free 1.5 mL microfuge tubes (Invitrogen by Thermo Fisher Scientific). The whole blood samples were then thawed, inspected for quality, and processed for genomic DNA (gDNA) extraction by using the PureLinkTM gDNA mini Kit (Thermo Fisher Scientific, Carlsbad, USA). Real-time polymerase chain reaction (PCR) was performed for HLA-B27 analysis using Applied BiosystemsTM TaqManTM assay (Thermo Fisher Scientific, Paisley, UK) as previously reported [7, 8] on an Applied BiosystemsTM QuantStudioTM 5 instrument (Thermo Fisher Scientific, USA) with 50°C for 2 min, 95°C for 10 min followed by 40 cycles with 95°C for 15 s and 62°C for 60 s including detection step at 62°C. TaqMan Universal Master Mix and TaqMan Assays were used for a 20 µL reaction volume. Cycle threshold (Ct) value for the HLA-B27 specific assays was compared with the Ct of the endogenous controls [globin and human growth hormone (hGH)]. Results have been marked as “undetermined” if Ct of the endogenous control was > 35 cycles. Patient acceptance of self-sampling was measured using the net promoter score (NPS).
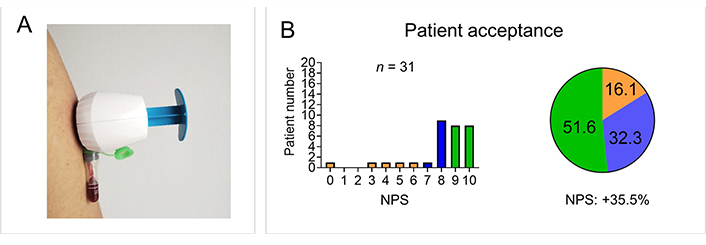
Patient self-sampling of capillary blood for HLA-B27 analysis. (A) An upper arm capillary blood self-sampling device of the type used in the study is shown; (B) patient acceptance for self-sampling is displayed as a bar graph and as a pie chart. The proportion of detractors (0–6) is shown in orange, the proportion of neutrals (7–8) in blue, and the proportion of promoters (9–10) in green
In total, 31/36 (88.6%) samples were received after the patient completion of unsupervised self-sampling as 5 patients did not mail their samples. HLA-B27 analysis was performed in all 31 samples and led to definite results in 29 of the samples (2/31 undetermined due to clotting). Concordance with the standard hospital-based venous analysis was 100% with 18/36 (50%) HLA-B27 positive results. Patient acceptance was high with an NPS of +35.5% [8.0/10 ± 2.3, mean ± standard deviation (SD)], supporting previous findings, (Figure 1B) [2–4]. The lack of return of samples could be reduced in future studies by reminding patients. The clotting of two samples in this study could be due to a handling error [the tube needs to be inverted in order to mix with blood with the additive or the tubes could have been overfilled with blood (not enough additive)]. Optional patient support with video consultations could decrease handling errors.
In conclusion, our study demonstrates for the first-time accuracy, feasibility, and high patient acceptance of unsupervised remote self-collection of capillary blood for HLA-B27 analysis in a cohort of patients with suspected axSpA. Thus, it may provide important groundwork for the implementation of screening and triage systems. Expanding capillary blood sampling to include c-reactive protein (CRP) and combining it with digital tools, such as smartphone-based symptom checkers, could increase diagnostic power and enable convenient and resource-efficient screening of axSpA patients to ultimately reduce axSpA diagnostic delay. Further studies are needed to assess barriers, facilitators, and the specific benefits of self-sampling in rheumatology.
axSpA: axial spondylarthritis
Ct: cycle threshold
HLA-B27: human leucocyte antigen-B27
We thank our patients for their willingness to participate in this study.
HL: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing—original draft, Writing—review & editing. IG: Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing. JK: Conceptualization, Data curation, Formal analysis, Funding acquisition, Supervision, Writing—original draft, Writing—review & editing.
JK has received research support from and has received consulting/speaker’s fees from Novartis Pharma GmbH and ABATON. The other authors declare that they have no conflicts of interest.
The study was approved by the regional ethics review board in Erlangen, Germany (Reg no. 21-357-B). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual respondents included in the study.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The data that support the findings of this study are available from the corresponding author JK, upon reasonable request.
The study was partially supported by Novartis Pharma GmbH, Nürnberg, Germany and the Deutsche Forschungsgemeinschaft (DFG – FOR 2886 “PANDORA” - Z to JK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2442
Download: 42
Times Cited: 0
Prakashini Mruthyunjaya ... Debashish Danda
Michael Schirmer ... Johannes D. Pallua
Diego Benavent ... Antonio Gómez-Centeno
Sumaira Farman ... Saira Elaine Anwer Khan
Carlos A. Guillén-Astete ... Mónica Vázquez-Díaz
Judy L. Seraphine, Alvin F. Wells
