Abstract
Background:
The United Kingdom (UK) currently employs a selective screening system for developmental dysplasia of the hip (DDH). Despite this, late presentation rates remain high. The aim of this study was to systematically review the available literature to gain an understanding of screening practices throughout the UK.
Methods:
A systematic review was conducted. Studies reporting DDH screening methods from the UK were included. The primary outcome measure was the method of ultrasound and clinical screening. Secondary outcomes were the treatment rate and late presentation rate. A narrative analysis was undertaken, as meta-analysis was felt to be inappropriate due to the differences between included studies.
Results:
Nine studies were eligible and included. There was significant variability in practice, with a variety of ultrasound techniques being used and a variety of staff members performing clinical screening. Treatment rate ranged from 16.4/1,000 to 0.8/1,000. Late presentation rate ranged from 1.28/1,000 to 0.27/1,000.
Discussion:
In spite of a national consensus statement, there is no standardised approach to clinical or ultrasound screening in the UK. A variety of different methods are used, which may explain the persistently high late presentation rate. A national system of quality control and a standardised screening process is recommended, with specialised training in the Graf method of ultrasound.
Keywords
DDH, screening, ultrasound, clinical screeningIntroduction
Rationale
Developmental dysplasia of the hip (DDH) is the most common orthopaedic disorder in newborns and is the leading cause of early-onset arthritis and total hip arthroplasty in young patients [1, 2]. Early detection and treatment of DDH can reduce the need for invasive surgical intervention and reduce long-term disability. Effective DDH screening is therefore essential to identify and treat cases as early as possible.
At present, the United Kingdom (UK) uses a selective screening model, with children with specific risk factors (breech presentation, multiple births, family history) or an abnormal clinical examination being invited for ultrasound screening. Clinical examination is performed at birth and repeated at 6–8 weeks in a primary care setting [3].
In 2022 the British Society for Children’s Orthopaedic Surgery (BSCOS) released a consensus statement regarding the management of DDH in the first three months of life [4]. They advocate for universal screening, but they also gave recommendations for selective screening within the current UK system. They recommend that the current risk factors be increased to include non congenital talipes equinovarus (CTEV) foot deformities and packaging disorders. They recommend that ultrasound should take place in a one-stop clinic, and that the Graf criteria of quality assurance should be adhered to. Those with clinical abnormalities on examination should have a scan within 2 weeks, and the examination itself should be performed by “expert” examiners with robust methods of quality assurance in place.
Objectives
This paper aims to evaluate screening in the UK by performing a systematic review of the relevant literature. This will provide an overview of the screening methodologies used. It can then be evaluated whether UK practice reflects that of the BSCOS consensus statement.
Materials and methods
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) compliant systematic review was conducted to gain an overview of current screening practice in the UK [5]. The Embase and Medlin databases were searched, along with the Cochrane Library.
Eligibility criteria
Study designs
Unpublished studies, guidelines, protocols, and editorials were not considered. Previous systematic reviews were also excluded. All other study designs were considered.
Types of participants
Exclusion criteria included studies with either serious or critical risk of bias.
Interventions
Interventions eligible for consideration were children who had undergone ultrasound screening for DDH in the UK. A full list of inclusion and exclusion criteria is detailed in Table 1.
Full inclusion and exclusion criteria
| Selection criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Language | English language | Non-English studies |
| Study type | All study designs | Systematic reviews |
| Population | Newborn populationHuman studiesUK based study | Children older than newbornCadaveric or animal studiesNon-UK study |
| Intervention | DDH screening | Not relevant to screening |
| Date | Studies after 2009 | Studies prior to 2010 |
| Quality | Clearly reported methodology | Serious or critical risk of bias |
| Availability | Full text available | Abstract only availableUnpublished studiesEditorials |
Outcomes of interest
The primary outcome measures of interest were the methods of ultrasound and clinical screening used. Secondary outcomes were the treatment rate and late presentation rate. The search strategy is detailed in Figure 1.
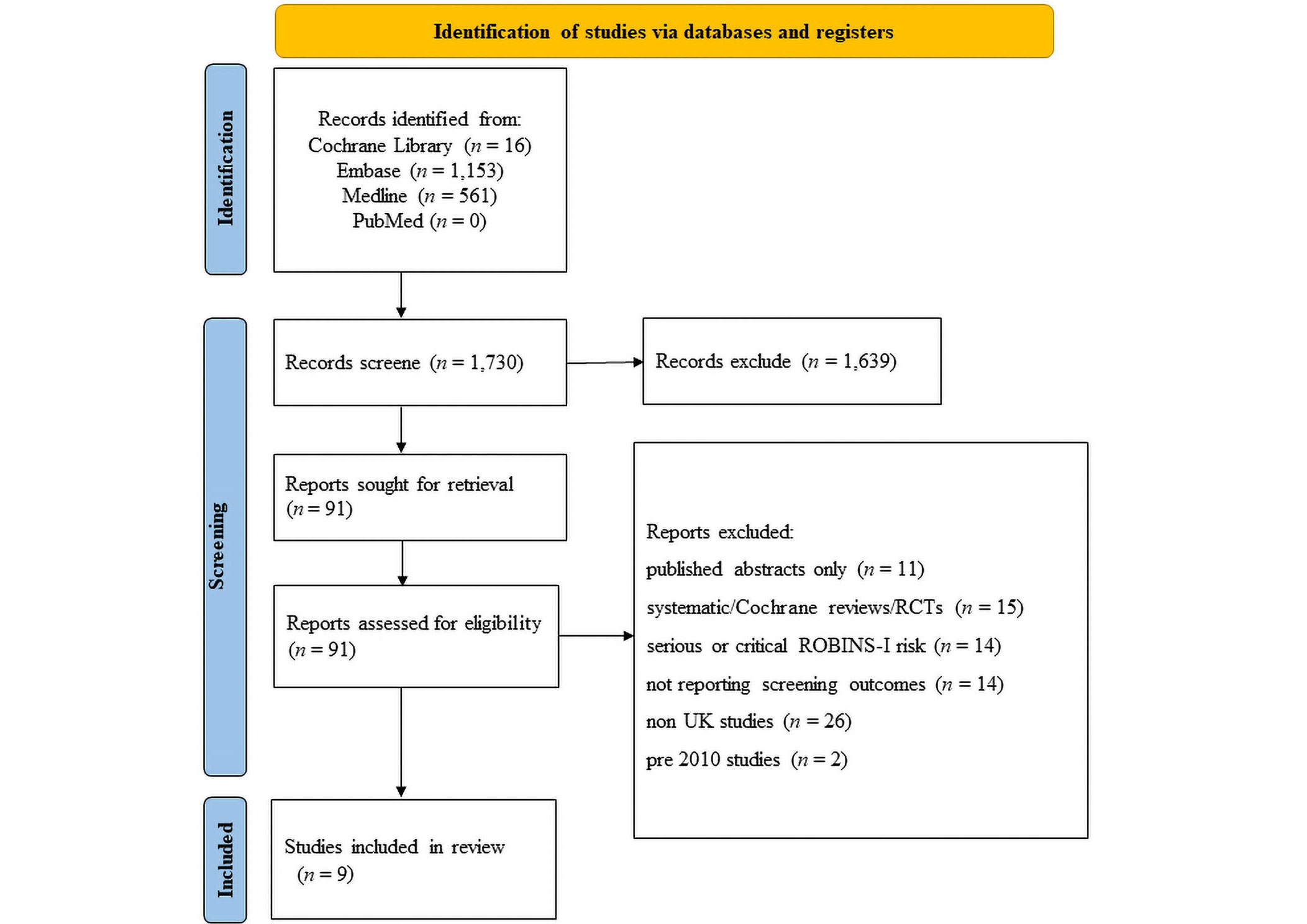
PRISMA flow diagram illustrating literature search. n: number. RCTs: randomized controlled trials; ROBINS-I: risk of bias in non-randomized studies of interventions
Search strategy
A systematic literature search was performed. The Embase, Medline, and PubMed databases, along with the Cochrane Library were searched. The search strategies and results are presented in Figure 1.
Selection process
The resulting papers were then manually searched to determine whether they met the inclusion criteria (Table 1). This was performed independently by 2 authors (Nicholas Birkett and Edward Karam). In the case of only one author including a paper, it was included in the review nonetheless.
Data collection process and synthesis methods
The methodologies and data extracted were reviewed. Meta-analysis was felt to be inappropriate due to the heterogeneity in both treatment modalities and outcome measurements. A narrative review was conducted to best answer the research question.
Risk of bias assessment
The ROBINS-I critical appraisal tool was used to evaluate the included studies for risk of bias [6]. The studies were then categorised as either low, moderate, serious, or critical risk.
Results
Study selection
A total of 1,730 studies were identified from the literature search (Medline, Embase, PubMed, and Cochrane Library). Ninety one studies were selected for full-text review following assessment of their abstracts. Of these, 9 met the inclusion criteria and were deemed eligible for this review. The full results of the literature search are presented in Figure 1.
Risk of bias in studies
The critical appraisal is summarised in Table 2, assessed using the ROBINS-I tool. The studies were all low or moderate risk.
Assessed risk of bias of included studies using the ROBINS-I tool
| Study | Type of bias | Overall bias risk | ||||||
|---|---|---|---|---|---|---|---|---|
| Confounding | Selection of participants | Classification of intervention | Deviations from intended interventions | Missing data | Measurement of outcomes | Selection of reported results | ||
| Afaq et al. [7] | Moderate | Moderate | Moderate | Low | Moderate | Low | Low | Moderate |
| Broadhurst et al. [8] | Low | Low | Low | Low | Low | Low | Low | Low |
| Choudry et al. [9] | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Clarke et al. [10] | Low | Low | Moderate | Low | Low | Moderate | Low | Moderate |
| Donnelly et al. [11] | Low | Low | Low | Low | Low | Low | Low | Low |
| McAllister et al. [12] | Low | Low | Low | Low | Low | Low | Low | Low |
| Talbot et al. [13] | Low | Low | Moderate | Low | Low | Low | Low | Low |
| Tyagi et al. [14] | Low | Moderate | Low | Low | Low | Low | Low | Moderate |
| Westacott et al. [15] | Low | Low | Low | Moderate | Moderate | Moderate | Low | Low |
Findings
The included studies highlighted the variability in screening practice throughout the UK, with a variety of reported clinical and ultrasound screening methods. All papers reported on selective screening, except for Westacott et al. [15], whose study compared universal with selective screening.
Primary outcome—ultrasound and clinical screening method
The ultrasound screening method was reported in 7/9 (78%) of papers. Five studies used either the Graf technique or a modification of it. Other methods used were Harcke, Terjesen, and Clarke. Often, these methods were combined and modified.
There was significant variability in the healthcare professional performing the ultrasound screening. Four studies did not report who performed the ultrasound screening. Other papers reported screening being performed by orthopaedic consultants, radiologists, sonographers, and physiotherapists. It is unclear from the papers whether these staff members had specific training or not.
Clinical examination was performed by a wide variety of staff. Most commonly, the examination was performed by either midwives or junior doctors in paediatrics. Other studies reported examinations by orthopaedic surgeons, physiotherapists, paediatricians, and health visitors.
Risk factors that prompted screening generally conformed with the UK newborn and infant physical examination (NIPE) guidelines [3]. Westacott et al. [15] included packaging disorders and non-CTEV foot disorders as risk factors.
Secondary outcomes
Treatment rates ranged from 16.4/1,000 to 0.8/1,000. The highest treatment rate was seen in the universal screening group in Westacott et al. [15], which is to be expected. Late presentation rates were poorly reported but ranged from 1.28/1,000 to 0.27/1,000. The definition of late presentation varied by study which made direct comparison difficult. It is worth noting that Westacott et al. [15] found 5 cases of delayed presentation in both their universal and selective screening groups, but all the cases in the universal group were due to administrative errors.
A summary of all the studies’ findings can be found in Table 3.
A summary of included studies and result
| Study | Number of patients | US method | Professional performing US | Professional performing clinical exam | Criteria for referral | Treatment rate | Late presentation rate |
|---|---|---|---|---|---|---|---|
| Afaq et al. [7] | 200 | Harcke method with Terjesen measurements | Consultant radiologist or sonographer | Junior doctor or midwife | Family history, breech, syndromic children, spinal or foot deformities, abnormal examination | Not reported | Not reported |
| Broadhurst et al. [8] | 3,635 | Not reported | Not reported | Not reported | Family history, breech presentation | Not reported | 1.28/1,000 |
| Choudry et al. [9] | 124 | Graf & Harcke | Orthopaedic consultant | Variable—junior doctor/midwife/advanced nurse practitioner | Family history, breech presentation, abnormal examination | 0.8/1,000 | 0.78/1,000 |
| Clarke et al. [10] | 20,344 | Clarke | Not reported | Paediatrician | Family history, breech presentation, foot deformities, abnormal examination | 7.2/1,000 | 0.34/1,000 |
| Donnelly et al. [11] | 75,856 | Graf | Not reported | Health visitors | Family history, breech presentation, abnormal examination | 8.5/1,000 | 0.42/1,000 |
| McAllister et al. [12] | 896,594 | Not reported | Physiotherapist/Physician | Physiotherapist/Physician | Family history, breech presentation, moulding abnormality, abnormal examination | Not reported | Not reported |
| Talbot et al. [13] | 2,984 | Modified Harcke (dynamic) & modified Graf (static) | Not reported | Orthopaedic surgeon | Family history, abnormal examination | 16.4/1,000 | Not reported |
| Tyagi et al. [14] | 3,618 | Graf | Consultant radiologist/radiographer | Maternity care professional | Family history, breech, multiple pregnancies, CTEV, high bodyweight females, abnormal examination | 3.31/1,000 | 0.27/1,000 |
| Westacott et al. [15] (selective group) | 18,053 | Graf | Radiographer or orthopaedic consultant | Junior doctor or general practitioner | Family history, breech presentation, multiple pregnancies, increased birth weight, non-CTEV foot deformities, packaging disorders | 2.3/1,000 | 0.28/1,000 |
| Westacott et al. [15] (universal group) | 10,015 | Graf | Radiographer or orthopaedic consultant | N/A—US screening alone | N/A—universal | 7.9/1,000 | 0.5/1,000 |
N/A: not applicable; US: ultrasound
Discussion
The included studies highlight the variability in DDH screening practice throughout the UK. Criteria for screening appear relatively similar and in keeping with NIPE guidelines, but work is required to expand the indications to include foot deformities and packaging disorders as per the BSCOS consensus statement. Most variability was noticed in ultrasound methodology and in the seniority or experience of those carrying out clinical examinations. It is clear from many of the studies that the important task of clinical examination is often carried out by a relatively inexperienced member of the team.
The late presentation rate of DDH in the UK has been estimated at 1.2/1,000 [16]. Countries with universal screening have reported late presentation rates ranging from 0 to 0.16/1,000 [17, 18]. The introduction of selective screening in the UK did not have any effect on late presentation rates, highlighting inconsistency in practice [8]. If the current system of selective screening in the UK is to continue for the foreseeable future, consistency and quality control are required at all levels of the process, from the initial assessment of the newborn to the ultrasound screening itself.
One essential area for improvement is education and training in the Graf method of ultrasound. The method is recommended but is often implemented in a modified fashion. The Graf method is successful when performed with the correct technique and the strict reporting criteria are adhered to. Modifications of the Graf method invalidate its efficacy, therefore widespread training in how to properly apply the method is required.
In conclusion, despite a national consensus statement, the UK literature demonstrates significant variability in practice. Significant change is required at a national level to standardise screening processes, with a national level of quality control to ensure that the highest quality care is being delivered. Specific training in the Graf ultrasound method is recommended, to ensure it is delivered correctly without modification.
Abbreviations
| BSCOS: | British Society for Children’s Orthopaedic Surgery |
| CTEV: | congenital talipes equinovarus |
| DDH: | developmental dysplasia of the hip |
| ROBINS-I: | risk of bias in non-randomized studies of interventions |
Declarations
Author contributions
NB: Conceptualisation, investigation, writing—original draft preparation, Writing—review & editing. EK: Conceptualisation, investigation, Writing—review & editing. DF and DP: Investigation, Writing—review & editing. CM: Conceptualisation, supervision, Writing—review & editing. All authors read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not Applicable.
Consent to participate
Not Applicable.
Consent to publication
Not Applicable.
Availability of data and materials
All data is from studies referenced in the manuscript.
Funding
Not Applicable.
Copyright
© The Author(s) 2024.