Affiliation:
1Osakidetza, OSI EE-Cruces, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
2Department of Medicine, Medicine and Nursery School, University of the Basque Country, 48903 Barakaldo, Spain
Email: fernando.perezruiz@osakidetza.eus
ORCID: https://orcid.org/0000-0002-5268-1894
Affiliation:
1Osakidetza, OSI EE-Cruces, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
ORCID: https://orcid.org/0000-0002-4983-5506
Affiliation:
4University of the Basque Country, Medicine and Nursing School, Cruces Teaching Unit, 48903 Barakaldo, Spain
ORCID: https://orcid.org/0009-0000-4140-8262
Affiliation:
1Osakidetza, OSI EE-Cruces, Cruces University Hospital, Rheumatology Division, 48903 Barakaldo, Spain
Affiliation:
6University Paris Cité, Inserm U1132, BIOSCAR, Department of rheumatology, DMU locomotion, Lariboisière Hospital, AP-HP, 75010 Paris, France
Affiliation:
7Division of Rheumatology, Department of Medicine, Spencer Fox Eccles School of Medicine, University of Utah, Salt Lake City, Utah 84132, USA
ORCID: https://orcid.org/0000-0002-1558-1202
Affiliation:
8Rheumatology Department, UMR 1131-Bioscar (Centre Viggo Petersen), CHU Lariboisière (APHP), University Paris, 75010 Paris, France
Explor Musculoskeletal Dis. 2024;2:375–383 DOI: https://doi.org/10.37349/emd.2024.00063
Received: May 16, 2024 Accepted: June 12, 2024 Published: September 10, 2024
Academic Editor: Jürgen Braun, Ruhr Universität Bochum, Germany
The article belongs to the special issue Calcium Pyrophosphate Deposition Disease
Aim: To ascertain the prevalence of calcium pyrophosphate arthritis (CPPA) at diagnosis and during follow-up of patients with gout.
Methods: Inception cohort of patients with gout prospectively recruited and followed-up from 1994–2023. Gout-case was defined as crystal-proved tophus or arthritis, or the presence of tophus plus double contour with ultrasonography. CPPA was defined as the presence of intra-leukocyte calcium pyrophosphate (CPP) crystals in synovial fluid (SF) and neat chondrocalcinosis in plain radiographs. Age, gender, time from onset of symptoms, number of flares, joint distribution, previous and prescribed treatments, colchicine prophylaxis, comorbidities, alcohol intake, use of diuretics, renal function, and previous vascular disease were available for analysis.
Results: A total of 1,544 patients with gout, with an average of 4-year follow-up, were available for analysis. CPPA was observed in 127/1,544 cases (8.2%). In 37/1,544 patients (2.4%) CPP and monosodium urate (MSU) crystals were observed in the same SF sample at gout diagnosis, and 90/1,544 (5.8%) showed CPP crystals apart from the diagnosis of gout. CPPA-gout cases had more flares per year, but no more frequent polyarticular distribution at baseline compared to non-CPPA-gout. CPPA-gout cases were older at baseline and showed lower renal function. Women, patients using diuretics, patients with hypertension, and those with previous vascular events showed CPPA more frequently. Multivariate analysis showed that only age and use of diuretics were independently associated with CPPA, as other variables apparently associated were dependent on aging. Interestingly, an analysis of the prevalence in the three decades available showed an increased CPPA diagnosis through time, probably associated with increased awareness of the association.
Conclusions: (1) CPPA is not infrequent in patients with gout; (2) it is associated with aging and diuretic use; (3) awareness of this association may increase the rate of diagnosis.
Gout is the most common inflammatory arthritis [1]. Calcium pyrophosphate disease (CPPD) is also frequent, especially in elders [2], clinically ranging from chondrocalcinosis (calcifications in joint cartilage in plain radiographs) to calcium pyrophosphate arthritis (CPPA), either acute CPPA (ACPPA) or and chronic CPPA (CCPPA). ACPPA was previously named in the literature as “pseudogout” [3] as ACPPA flares are clinically undistinguishable from gout flares.
Persistence of gout flares in patients with gout even after a proper treat-to-target treatment approach was observed during long-term open-label extension of clinical trials to evaluate urate-lowering medications [4]. This raises the question of whether the coexistence of a disease with similar clinical characteristics at work, namely ACPPA, could be inducing what in some cases could be considered treatment-failure gout [5]. In addition, a definite diagnosis of both conditions, gout, and CPPA, would oblige us to consider investigating etiological causes of CPPA, such as hypomagnesemia, hyperparathyroidism, or iron storage [6, 7], and also considering long-term colchicine treatment.
We performed this analysis in order to ascertain the prevalence of CPPA at diagnosis and during follow-up of patients with gout.
Data were obtained from an inception cohort of patients with gout prospectively followed-up from Jan 1994 to Dec 2023 (30-year recruitment). Gout-case was defined by the presence of monosodium urate (MSU) crystals in synovial fluid (SF) samples or material aspirated from a tophus, using a polarized light microscope with compensating first order red filter [8], or findings of a tophus plus a double contour sign in hyaline cartilage in ultrasonographic examination [2, 9]. CPPA was defined as the presence of intra-leukocyte calcium pyrophosphate (CPP) crystals in SF plus neat chondrocalcinosis in the same or a different joint in plain radiographs [2]. Age, gender, flares in the year previous to entering into the follow-up cohort, gout evaluation, joint distribution (monoarticular, oligoarticular, polyarticular), previous and prescribed urate-lowering therapy (ULT), prophylaxis with colchicine, alcohol intake (over 15 g/day), comorbidities (hypertension, hyperlipidemia, and diabetes were considered according to varying international definitions through decades), previous vascular event, baseline serum urate, renal function (estimated glomerular filtration rate using Cockroft-Gault, MDRD, CKD, and CKD-EPI formulae through time), use of diuretics, and previous renal lithiasis were available for analysis. Analysis was made using an institutional license of the statistical package IBM SPSS V29.0. Results are shown as mean ± standard deviation (median, interquartile range) for continuous variables and percentage for discrete variables. Variables showing P < 0.2 in bivariate analysis were selected for multivariate analysis using binary logistic regression.
One thousand, five hundred and forty-four patients with gout were available for analysis; the mean follow-up was 43 ± 47 months (median 24 months, interquartile range 12–60 months). CPPA was present in 127/1,544 (8.2%) patients. Only 3/127 (2.4%) had a previous diagnosis of CPPA, all of them were previous diagnoses from our rheumatology division where in 37/1,544 patients (2.4%) a simultaneous diagnosis of gout and CPPA was made due to the presence of both CPP and MSU crystals were observed in the same synovial sample (Figure 1) at first evaluation for gout, and finally, 90/1,544 (5.8%) patients showed CPP crystals apart from the initial diagnosis of gout. Most of the CPPA-gout cases, 60/90 (66.6%), were identified within the first 5-year period of follow-up.
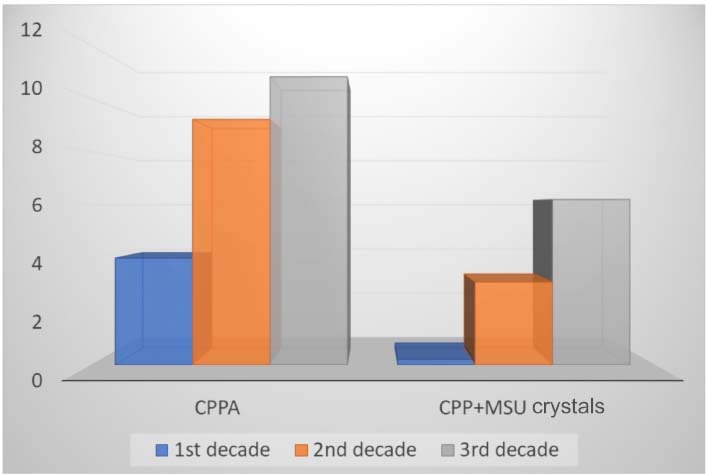
Frequency (%) through three decades of patients with a diagnosis of calcium pyrophosphate arthritis (CPPA). CPP: calcium pyrophosphate; MSU: monosodium urate
Analysis of patients with both PPA and gout at gout diagnosis (both crystals in SF) vs. patients with a CPPA diagnosis after the gout diagnosis showed no difference in any of the variables analyzed (data not shown).
CPPA-gout cases are referred numerically, but not significantly, with more flares per year in the previous year to gout diagnosis, but no more frequent polyarticular joint involvement at baseline than non-CPPA (Table 1). In bivariate analysis, CPPA-gout cases were older at baseline, and women were more commonly affected than men. Patients using diuretics, with hypertension, and previous vascular events, were more frequently affected. Alcohol intake over 15 g/day was apparently protective, as the rate of CPPA was lower (Table 1) than in patients with low or no alcohol intake.
Variables analyzed for association with calcium pyrophosphate arthritis (CPPA) in patients with gout
| Variable | CPPA (%) | Non-CPPA (%) | P |
|---|---|---|---|
| Age (years) | 72 ± 11 | 61 ± 13 | < 0.001 |
| Time from onset (years) | 6.55 ± 7.02 | 6.61 ± 8.04 | 0.926 |
| Flares (previous year) | 3.59 ± 4.10 | 3.82 ± 5.95 | 0.556 |
| GFRe (mL/min) | 73 ± 29 | 62 ± 26 | < 0.001 |
| Serum urate (mg/dL) | 9.18 ± 1.63 | 9.13 ± 1.46 | 0.349 |
| Gender (female) | 20/127 (15.7) | 128/1,417 (9.0) | 0.014 |
| Polyarticular involvement | 46/127 (36.2) | 491/1,417 (34.7) | 0.772 |
| Tophi | 43/127 (33.9) | 452/1,417 (31.9) | 0.650 |
| Previous ULT | 61/127 (48.0) | 613/1,417 (43.3) | 0.299 |
| ULT febuxostat | 18/127 (14.2) | 142/1,417 (10,0) | 0.086 |
| Colchicine for prophylaxis | 96/110 (87.3) | 1012/1,184 (85.4) | 0.607 |
| Diuretic use | 72/127 (56.7) | 454/1,417 (32.0) | < 0.001 |
| Alcohol intake (> 15g/day) | 26/127 (20.5) | 453/1,417 (32.0) | 0.007 |
| Hypertension | 102/127 (80.3) | 782/1,417 (55.2) | < 0.001 |
| Hyperlipidemia | 63/127 (49.6) | 778/1,417 (54.9) | 0.250 |
| Diabetes | 34/127 (26.8) | 354/1,417 (25.0) | 0.656 |
| Vascular disease | 64/127 (50.4) | 450/1,417 (31.8) | < 0.001 |
| Urolithiasis | 8/127 (6.3) | 101/1,417 (7.1) | 0.725 |
GFRe: glomerular filtration rate estimated; ULT: urate-lowering therapy
Aging was associated with a higher frequency of diuretic use, febuxostat prescription, vascular disease, and hypertension (data not shown). Aging was also associated with lower GFRe and lower frequency of alcohol intake (data not shown), as expected in the general population. Therefore, multivariate analysis was performed to ascertain which variables were independently associated with CPPA. In multivariate analysis, only age and diuretics remained independently associated with CPPA (Table 2).
Multivariate analysis for variables associated with calcium pyrophosphate arthritis (CPPA) in gout patients
| Variable | Exp(B) | 95% LCIL | 95% UCIL | P |
|---|---|---|---|---|
| Age (years) | 1.048 | 1.030 | 1.066 | < 0.001 |
| Diuretic use | 1.972 | 1.277 | 3.045 | 0.002 |
| GFRe (mL/min) | 1.008 | 0.999 | 1.017 | 0.072 |
| Hypertension | 1.257 | 0.716 | 2.208 | 0.426 |
| Vascular events | 1.039 | 0.675 | 1.597 | 0.863 |
| Alcohol (> 15 g/day) | 0.990 | 0.605 | 1.620 | 0.969 |
| Gender (female) | 1.052 | 0.640 | 1.830 | 0.861 |
| Febuxostat use | 1.030 | 0.581 | 1.828 | 0.918 |
Exp(B): odds ratio, predicted change in odds for a unit increase in the predictor; LCIL: lower confidence interval limit; UCIL: upper confidence interval limit; GFRe: glomerular filtration rate estimated
The analysis of the prevalence in the three decades available showed a progressive increase in CPPA diagnosis: 18/454 (4.0%), 50/543 (9.2%), and 59/547 (10.8%) for the first, second, and third decade respectively. The same pattern appeared for finding CPP and MSU crystals in the same sample: 1/436 (0.2%), 13/420 (3.1%), and 23/368 (6.2%), for the first, second, and third decades respectively (Figure 1).
Gout and CPPA are the most frequent causes of inflammatory arthritis [1, 2], and their prevalence increases with age [10, 11] as the prevalence of the main causal factors, namely hyperuricemia and chondrocalcinosis respectively, also increases with age [12, 13].
CPP crystals have been occasionally demonstrated in 0.66% of SF samples of patients with a previous clinical diagnosis of gout [14], but in up to 13/49 (26.5%) with a transversal study of crystal examination of SF samples [15]. Inversely, MSU crystals were observed in 0.80% of patients with a previous clinical diagnosis of acute CPPA [14]. The coexistence of gout and “pseudogout” (acute CPPA) was reported in an analysis of The Health Improvement Network (THIN) database [7]. The authors found 795 patients with a diagnostic coding of “pseudogout”, but they excluded 310 additional patients diagnosed with both “pseudogout” and gout. Taken together, the prevalence of both conditions in that database would be 310/1,105 (28.05%), which may mean that gout and “pseudogout” flares, undistinguishable from the clinical point of view, could be frequently misdiagnosed and miscoded.
Indeed, the only way to properly diagnose an episode of acute arthritis in patients showing both hyperuricemia and chondrocalcinosis is to obtain a SF sample and search for crystals in a microscope. Therefore, a gout flare could be considered as “pseudogout” in a patient showing chondrocalcinosis in radiographs, or a gout flare in a patient with acute CPPA showing hyperuricemia. To make it more complicated, we have demonstrated that the coexistence of both crystals in the same SF is not infrequent in patients with both gout and chondrocalcinosis.
Most of the CPPA diagnoses were made in the first five years of follow-up. The limited follow-up of patients, a mean of 43 months, with only 25% of patients with a follow-up over 60 months (upper interquartile range), and the higher probability of having flares during initiation of ULT may explain this result and even underestimate the cumulated prevalence of CPPA in gout. Therefore, the development of joint inflammation during long-term follow-up of patients with gout properly treated to target deserves further investigation, and especially to rule-out CPPA.
Only aging and diuretic use, in this prospective cohort, were independently associated with CPPD-gout. Aging is associated with gout presentation in women [16], increased prevalence of hypertension and vascular events [17], and declining renal function, therefore explaining the association of CPPA with variables related to aging. In our cohort, febuxostat use was also associated with increased aging, as febuxostat is easier to handle than allopurinol in patients with comorbid conditions, especially patients with CKD [18].
Alcohol intake was apparently “protective” in bivariate analysis, but this association was inversely related to aging. Although our results are contrary to data in the general population in a neighbor European country [19], a decrease in alcohol intake with aging in the USA population has been observed [20]. It is plausible that in diseased patients, as it may happen in a hospital-based setting like ours, the rate of alcohol intake would decrease with aging and increasing comorbidity and could differ from that of the general population.
Chondrocalcinosis (radiography finding) and CPPA (clinical episode) have been associated with the use of either thiazide or loop diuretics [6, 7]. Diuretics, especially thiazide diuretics, may induce hypomagnesemia, which is associated with an increased risk of CPPA [6]. There is a direct association between serum magnesium levels and the risk of radiographic chondrocalcinosis [21].
Patients with the coexistence of both crystals in SF, or mixed crystal arthritis, did not differ clinically from patients with a previous diagnosis of gout showing only urate crystals at the moment, probably indicating that CPPA had a small impact on the patient’s clinical profile. No quantitative analysis of the presence of each kind of crystal was performed to discertain which of them was mainly involved in the inflammatory process. We personally think that the presence of both MSU and CPP crystals intracellularly would suggest a diagnosis of mixed crystal disease as both are probably causing neutrophil activation and sharing an inflammatory role.
The coexistence of both CPPA and gout may have implications, in addition to diagnosis, as previously commented, for treatment. Colchicine is recommended for the treatment of CPPA in order to prevent flares [22]. An increased risk of cardiovascular events has been reported to be associated with CPPA [23] and CPPA flares [24]. Colchicine is an anti-inflammatory agent shown to be useful and was recently labeled in the USA, for the prevention of cardiovascular events [25]. Therefore, prolongation of colchicine treatment could be considered in patients with CPPD-gout, especially those at high risk of cardiovascular events due to associated comorbid conditions [26]. Our results also suggest that chondrocalcinosis should be excluded during the recruitment of patients with gout for clinical trials, at least those aged or using diuretics.
Interestingly, we found an increasing frequency of CPPD and the presence of coexisting crystals in SF samples over decades. It has been shown that increasing experience is not associated with overconfidence [27], so a higher rating is to be expected in the first decades associated with misclassification of crystal findings in SF.
This study has inherent strengths. Gout and CPPA diagnoses were based on crystal-based gold standards, as classification criteria for gout [28] and CPPA [29] are quite recent. For CPPA, an even more stringent crystal plus plain X-ray diagnosis was needed [2] as the USA was not available for daily clinical practice at the initial stages of the cohort. Therefore, this very stringent definition may underestimate the real prevalence of CPPA. In addition, all patients were personally and prospectively followed-up by the same physician (Fernando Perez-Ruiz), thus avoiding diversity of criteria.
Nevertheless, some limitations should be considered. The study shows an increasing diagnosis rate of both CPPA and the coexistence of MSU and CPP crystals in the same SF sample through decades, signaling that underdiagnosis of CPPA was plausible at least during the first decade [27]. On the other hand, a third-level, hospital-based population may have an increased rate of coexistence of both diseases, as we are dealing with an aging population with frequent comorbid conditions, and frequently treated with diuretics, a risk factor for both gout [30] and CPPD [6]. Coexistence of other diseases with gout involving, such as diffuse idiopathic skeletal hyperostosis has not been shown either to have an impact on the clinical profile of gout in our patients, but the severity of a hospital-based population may minimize the effect [31]. Finally, as patients who were properly controlled to target serum urate, asymptomatic, and with no significant comorbidity needing tertiary care were discharged to primary care, the follow-up was limited in time for a considerable number of patients.
In conclusion, CPPA is not infrequent in patients with gout, and MSU and CPP crystals may coexist. CPPA in patients with gout is only independently associated with aging and diuretic use. Awareness of this association increases the rate of diagnosis.
ACPPA: acute calcium pyrophosphate arthritis
CPP: calcium pyrophosphate
CPPA: calcium pyrophosphate arthritis
CPPD: calcium pyrophosphate disease
MSU: monosodium urate
SF: synovial fluid
FPR will be always in debt to Ms. Concepción Eizaguirre and Mrs. Begoña Eizaguirre for their outstanding contribution to education.
FPR: Conceptualization, Investigation, Formal analysis, Writing—original draft, Writing—review & editing, Funding acquisition. MdCMC, JASdB, NS, HKE, and FL: Conceptualization, Writing—review & editing. AMHB: Writing—original draft, Validation, Writing—review & editing, Formal analysis. Nuria PH and Nerea PH: Writing—original draft, Formal analysis, Writing—review & editing. All authors read and approved the submitted version.
Fernando Perez-Ruiz: advisor for Arthrosi, Horizon, LG, Protalix, and SOBI, speaker for Menarini. Naomi Schlesinger: advisor or review panel member: Horizon Pharma, Novartis, Sobi, Protalix, Arthrosi, and Shanton. Fernando Perez-Ruiz is Editor-in-chief of the journal Exploration of Musculoskeletal Diseases, Naomi Schlesinger is Associate Editors, and Maria del Consuelo Modesto-Caballero is Editorial Board Member, none of them was involved in the decision-making or review process for this manuscript. The other authors declare that they have no conflicts of interest.
This study was approved by the Ethics Committee of OSI EEC at Cruces University Hospital [CEIC-E03/45].
Informed consent to participate in the study was obtained from all participants, and the patient’s information sheet was supervised and approved by the Ethics Committee of OSI EEC at Cruces University Hospital.
Not applicable.
The dataset is available on request to authors and with previous approval by the Ethics Committee of OSI EEC.
This study was funded by the Cruces Rheumatology Association [24/01].
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
John F. Hoy ... Xavier C. Simcock
Michael Schirmer, Johannes Dominikus Pallua
Anna J. Turlej, Angelo L. Gaffo
Maria L. Voulgari, Herbert Kellner
Gamze Dilek ... Kemal Nas
Ebru Atalar, Hatice Bodur
