Abstract
The temporomandibular joint (TMJ) involvement is an underestimated feature of juvenile idiopathic arthritis (JIA) since it is usually asymptomatic at presentation for an undeterminable time. Late diagnosis of TMJ arthritis in JIA patients leads to delayed treatment, long-term orofacial disturbances, and impaired health-related quality of life (HRQOL). Therefore, the detection of TMJ involvement is fundamental and represents a challenge. This perspective presents state-of-the-art current initiatives and available tools for early diagnosis of TMJ arthritis in children with JIA. Standardized protocols and multidisciplinary efforts for improving clinical skills in the assessment of TMJ in JIA are presented and commented on. An overview of imaging efforts for early detection of TMJ involvement in JIA is also provided, with a critical review of the advantages and limitations of different techniques, imaging protocols, and scoring systems. The perspective offers insights into the correct use and improvement of available and potential tools for early identification of TMJ arthritis in JIA subjects who deserve timely multidisciplinary treatment, avoiding both underestimation and over-diagnosis of TMJ arthritis in routine clinical practice.
Keywords
Juvenile arthritis, temporomandibular joint, imaging, clinical examination, early diagnosisIntroduction
First described by Thomas Phaire in the first English-language textbook of pediatrics as a group of conditions characterized by “stifnes or starcknenes of limes” (1545), mentioned by Cornil in 1864 and further detailed in publications by Mayer Saül Diamantberger (1891) and George Frederic Still (1897) [1–3], juvenile idiopathic arthritis (JIA) encompasses a heterogeneous set of chronic inflammatory diseases in children, essentially identified by chronic inflammatory arthritis with onset before the age of 16 years and of unknown origin. Current naming and classification, though under criticism in the recent decades, are due to the definitions revised in 1997 and 2001 by the International League of Associations for Rheumatology (ILAR), which agreed to merge the terms “juvenile rheumatoid arthritis” and “juvenile chronic arthritis”, respectively adopted by the North-American and the European researchers. ILAR classification criteria define seven JIA subtypes, depending on the association with systemic features, the number of joints affected in the first six months of the disease and its course, the presence/absence of IgM rheumatoid factor (RF) on at least two occasions at least 3 months apart, the occurrence of psoriasis or psoriatic features, and the axial and entheseal involvement, namely: the systemic, the oligoarticular (persistent or extended, based on the number of joints cumulatively involved after the first 6 months of disease), the polyarticular RF-negative, the polyarticular RF-positive, the psoriatic, the enthesitis-related arthritis (ERA), and the undifferentiated arthritis subtype [4–8]. The estimated incidence of JIA is 1.3–22.6 per 100,000, higher among people of White European ancestry than among those of Asian ancestry [9]. JIA typically presents with joint inflammation, swelling, morning stiffness, pain, and limited mobility involving one or more joints [10]. Though the distribution of joints involved may vary across the different JIA subtypes, the most frequently affected joint is the knee, followed by the ankle, the wrist, the second and third proximal interphalangeal joints, the second and third metacarpophalangeal joints, the elbow, the cervical spine, and the hip. The sternoclavicular, acromioclavicular, distal interphalangeal, and foot interphalangeal joints are rarely affected in children with JIA [9–11].
Arthritis of the temporomandibular joint (TMJ) does not typically manifest with joint swelling or pain. Physical exam findings of TMJs can be detectable late in the disease process when the bone growth has been altered by the arthritis. This makes it difficult to capture TMJ involvement at the early stages, which conversely would be of much benefit for avoiding irreversible damage and impaired functions. Further, TMJ has great remodeling capabilities during developmental ages, which reinforces the urgency of harnessing the full potential rescue of compromised joints [12, 13].
For many years, TMJ has been regarded as the forgotten joint by pediatric rheumatologists, whereas its involvement in JIA attracted a lot of interest among dentists, with a substantial niche focus on the repercussions on mandibular growth and the subsequent abnormal secondary occlusion. Nonetheless, in the past two decades, there has been growing evidence of high frequent involvement of TMJ in JIA patients. Depending on the diagnostic method used (clinical and/or imaging-based), the examiner specialty (pediatrician, rheumatologist, orthodontist, maxillo-facial surgeon), the JIA subtype and population investigated, studies have reported from forty to ninety-six percent of JIA patients with TMJ arthritis along the disease course [14–16].
Early recognition for timely and appropriate treatment is the key to effective multidisciplinary management of TMJ in JIA and the reduction of irreversible damage and subjective discomfort. In the present perspective, diagnostic clinical and imaging tools currently available for early detection of TMJ involvement in JIA will be presented and discussed.
Why TMJ involvement is relevant in patients with JIA?
The complex formed by the TMJs and the masticatory muscles allows the mandible to move up and down, side to side, and forward and back. The proper alignment of the mandible and the TMJs is also pivotal for several smooth actions that we unconsciously perform many times a day, including chewing, talking, yawning, and swallowing.
The peculiar structure of TMJ distinguishes it from the other joints, since it consists of a bilateral synovial diarthrosis, defined as “ginglymoarthrodial joint”, with fibrous cartilage covering the articular surface. Occlusion and masticatory muscles are the main drivers of the condylar position. The growth of the mandible, the dentition, and the facial skeleton are essentially due to major growth nuclei of the lower jaw, which are symmetrically located in the condyles. In particular, their superficial proliferative areas lay very close to the synovial membrane in the articular cartilage. Hence, in contrast with the peripheral joints, where the distance between the synovial membrane and the growing areas is rather wide, in TMJs, any inflammatory process of the synovium immediately affects these delicate zones, and may quickly alter the physiological growth [17–19]. Of note, the most intense mandible growth occurs in the first 6 years of age, whereas its later potential development drops to 15% up to 21 years of age. Therefore, the younger the child and the longer the period in which growth disturbance occurs, the greater the impairment of morphologic development [20].
Persistent synovitis of the TMJ in children with JIA may lead to reduced mouth opening, pain, and abnormal craniomandibular growth. When bilateral, depending on the child’s age, micrognathia, dental malocclusion, and anterior open bite are the most evident clinical features. On the contrary, unilateral involvement is responsible for facial asymmetry, as prominent as the younger the patient at its onset. Morphological alterations can be associated with muscle functional impairment and pain, resulting in difficulty with proper oral hygiene and an increased risk of dental caries [21]. Moreover, a recent wide cross-sectional study, including 3,343 children with JIA, documented that JIA patients with TMJ involvement experience higher levels of disability and disease activity, and impaired health-related quality of life (HRQOL), compared to JIA patients without TMJ involvement and healthy children [22, 23].
How to thoroughly and feasibly assess TMJ in JIA patients on clinical examination?
The complete joint examination is the essential clinical tool for the detection of joint swelling, tenderness, pain on motion, and limited range of motion in patients with JIA, and includes the evaluation of TMJs (Figure 1).
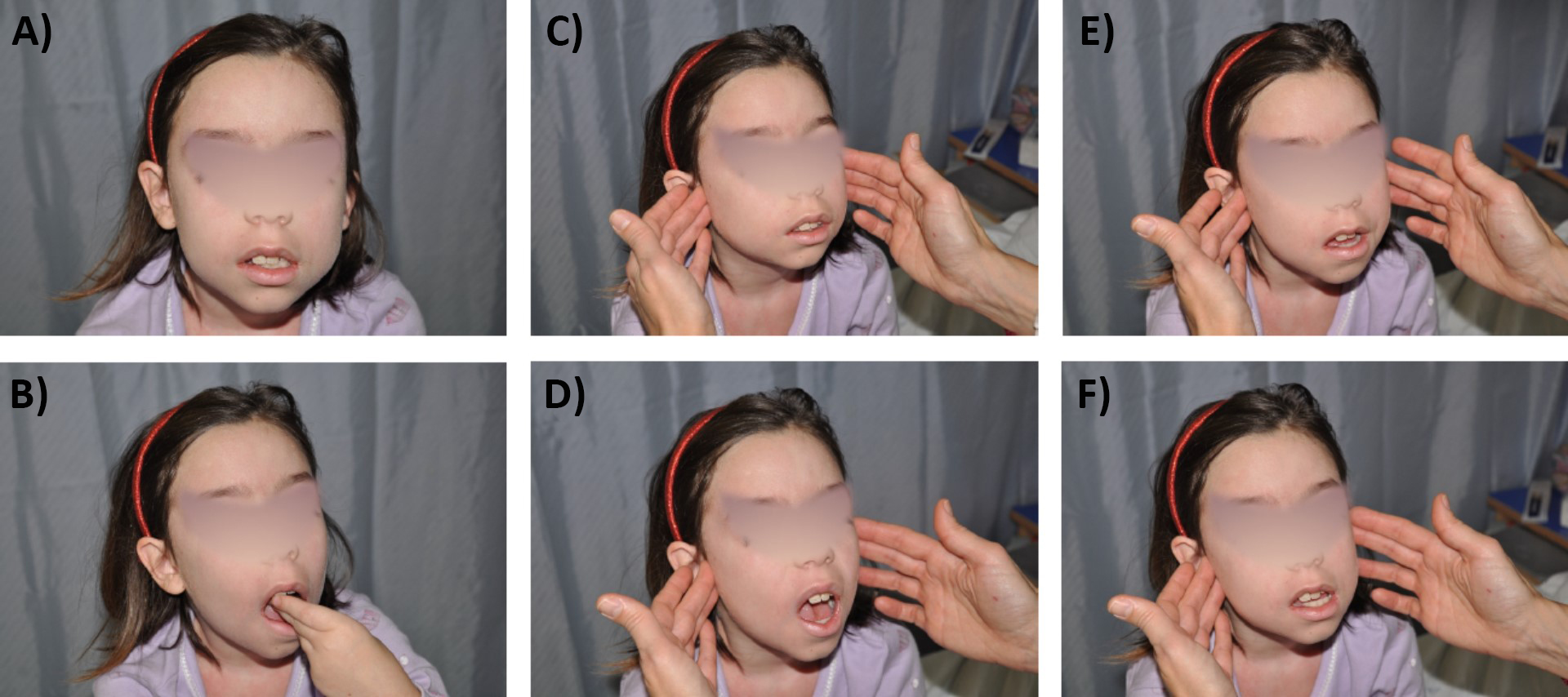
Clinical examination of the temporomandibular joint. Inspection in neutral position at mouth closed (A) and mouth opening (B), asking “Place your own 3 fingers vertically in your mouth” for assessing maximal mouth opening. Temporomandibular joint (TMJ) palpation with closed mouth (C), open mouth (D), extension (E), and lateral deviation (F) (at both sides), for assessing deviation of the jaw, small jaw micrognathia, clicks
However, TMJ swelling is not clinically detectable and JIA patients rarely report complaints or present limitations of TMJ, unless at later stages. Chronic inflammation of the TMJ can result in subjective symptoms and clinical signs, such as pain on movement and upon palpation, sounds upon mouth opening like cracks and crepitations, reduced maximum interincisal opening, limited movements and jaw deviation. Mandibular growth may be unilateral or bilaterally impaired in case of affection of one or both TMJ, at a variable degree depending on the child’s age at onset of TMJ disease and TMJ disease duration, as already mentioned [24, 25].
Indices for the anamnestic dysfunction (Ai) and the clinical dysfunction (Di) were developed by Helkimo in 1974 to identify the degree of severity of TMJ dysfunction [26]. The Helkimo index consists of three different levels, corresponding to three sub-indices: the anamnesis index, derived from the history taking, with the reporting of symptoms of dysfunction in the masticatory system by the individuals; the clinical dysfunction index, which expresses the functionality of the masticatory system; and the occlusal index, which takes into account the complete analysis of the individual’s dental occlusion, with assignment of a score of 0, 1, or 5 points for number of teeth, number of teeth in occlusion, occlusal interference between centric relation and centric occlusion and joint interference.
Helkimo’s indices have seldom been used for investigating TMJ involvement in JIA patients. Some researchers applied them to study the association between initial clinical TMJ findings and the severity of TMJ involvement over time [27, 28]. Another study measured the association between clinical TMJ findings at the initial examination of JIA patients and the severity of TMJ arthritis, assessed clinically through the Helkimo’s indices and on imaging through TMJ magnetic resonance imaging (MRI) [29]. However, Helkimo’s index is considered highly subjective for two main reasons: there are no definitions for calculating the anamnesis and occlusal components of the index; the examination of the three components does not merge into a single numeric value. Hence, its interpretation and use for comparison in the same subject over time and in different subjects remain unclear [30].
Other authors explored the performance in JIA of detailed examination protocol for the detection of temporomandibular disorders (TMD), i.e., Diagnostic Criteria/Temporomandibular Disorders (DC/TMD) protocol [31, 32]. The updated protocol for children and adolescents registers the presence or absence of pain or headache in the 30 days prior to the examination and the location of pain; it includes the path of jaw opening and jaw deviation (corrected or uncorrected) in maximum opening, measure of maximum mouth opening, right laterality, left laterality, protrusion, presence of pain associated with these movements, with the specification of location (muscular, articular), pain on muscle palpation, pain and noise on joint palpation and auscultation. Maximum mouth opening is considered limited when below 36 mm in adolescents, as in adults, and < 32 mm in children. Laterality and protrusion movements can be studied only in adolescents (≥ 10 years): laterality movements are considered limited when below 8 mm, whereas protrusion movements when below 5 mm. The DC/TMD protocol is very detailed and complete, but, similarly to Helkimo’s indices, it is complex, requires specific orthodontic training, is time-consuming for completion, and does not specifically focus on orofacial manifestations of JIA [25].
In the recent years the Temporomandibular Joint Juvenile Arthritis Working Group (TMJaw), an independent group of international specialists in multiple areas, reported recommendations for standardized orofacial examination in patients with JIA that can be performed by pediatric rheumatologists as well as dentists and maxillofacial-surgeons in daily practice [33, 34]. These recommendations emphasize the importance of history taking and clinical examination, and provide clear explanation and illustration for a < 3 minutes examination (Figure 2).
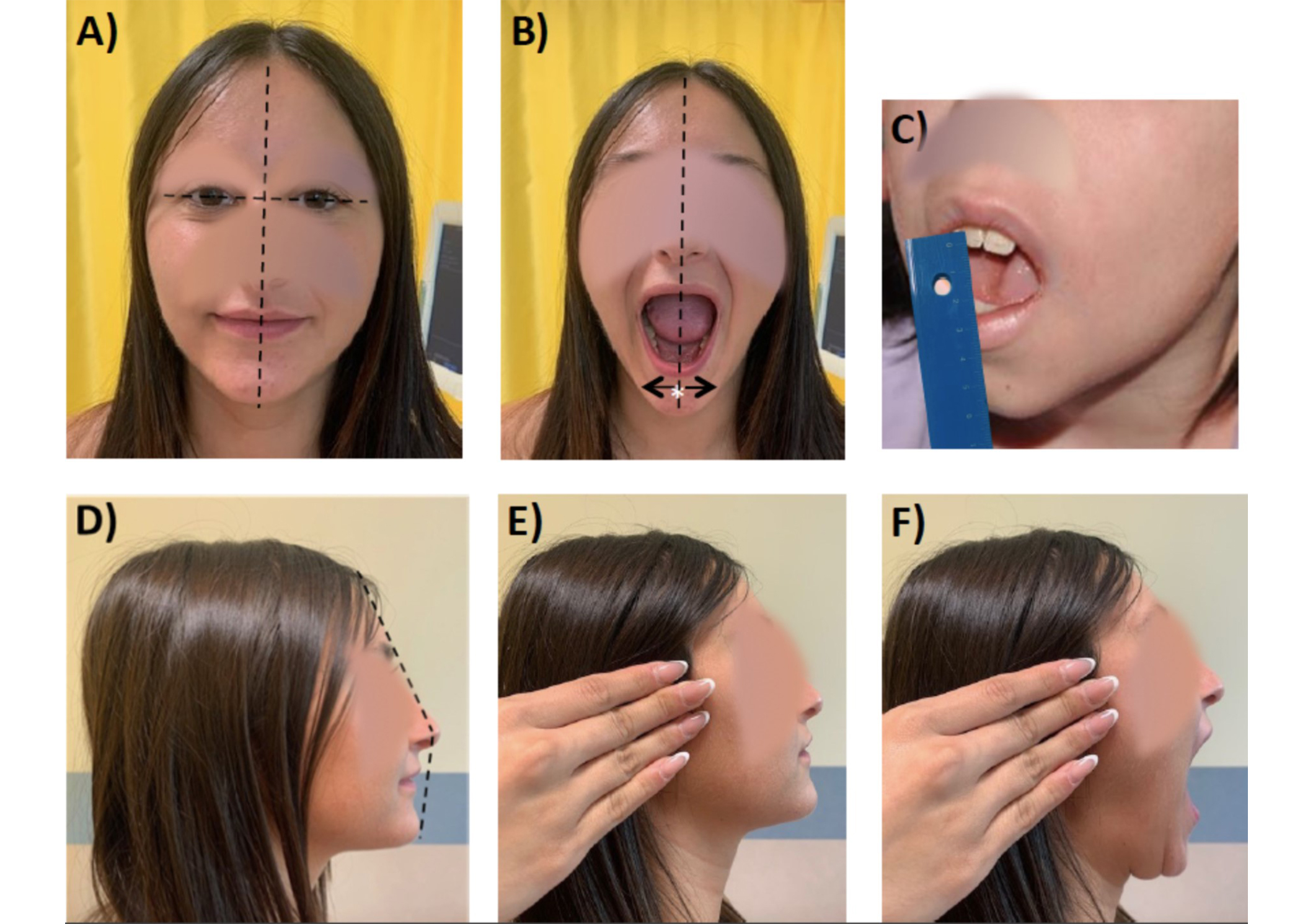
Clinical examination according to the Standardized Short Screening Protocol for temporomandibular joint (TMJ) in juvenile idiopathic arthritis (JIA). (A) Assessment of facial symmetry: an ideal horizontal line passing through the pupils (interpupillary line) is perpendicularly crossed by a longitudinal line passing through the glabella, which defines the facial midline; (B) mandibular deviation (to be performed also at maximal mouth opening), the white asterisk “*” indicates the chin point; (C) maximal mouth opening capacity; (D) assessment of facial profile (e.g., convexity); (E) TMJ palpation with closed mouth; (F) TMJ palpation with open mouth
The TMJaw group also provided standardized terminology, with the distinction of active inflammatory arthritis (TMJ arthritis) from damage/dysfunction resulting from previous disease (TMJ involvement) [35]. Data from a Danish JIA cohort study revealed that at least one orofacial symptom and at least one orofacial dysfunction at baseline examination similar to the proposed short screening protocol were associated with hazard ratio (HR) 2.2 and 3.3, respectively, of dentofacial deformities at 36 months follow-up [36]. Hence, this tool appears to be reliable and feasible in the routine clinical setting of different specialties.
Imaging of TMJ in JIA: which are the best-suited modalities for early detection of TMJ arthritis?
Conventional radiology
For a long time, serial cephalometric radiographs or tracings taken for the same patient at different time intervals have been the traditional radiologic technique for assessing mandibular growth during childhood and puberty in children with JIA and TMJ arthritis. However, the assessment of growth in two-dimensional (2D) radiographs modality is hampered by different magnification and positioning, which can lead to distortion and overlapping of 3D structures [37, 38]. In addition, no information on active synovitis at the TMJs can be retrieved.
A low-cost medical imaging method is represented by orthopantomography (OPT), which is characterized by lower radiation exposure and wide availability in dental offices. OPT allows the detection of several structural changes, including condylar asymmetry, erosions, osteophytes, and surface flattening. Similarly to cephalograms, overlapping of anatomical structures and other potential artifacts reduce its ability in the detection of very small lesions [39]. OPT was used in some studies for investigating condylar, ramal, and condylar plus ramal asymmetries. In these studies, the asymmetry index, calculated as a ratio of condylar and ramal length, proved to be a reliable reference even with different magnification sheets [39, 40] (Figure 3). Further, some investigators compared OPT with MRI with gadolinium in JIA patients and found that abnormal condyle morphology, accentuated antegonial notch, or short ramus, and condyle unit at OPT were significantly associated with TMJ synovitis detected on MRI [39]. Therefore, OPT could be considered for routinely assessment of JIA patients, at the price of insufficient sensitivity for fine bone alterations, as a screening tool for deciding on referral to orthodontists, or, vice versa, to rheumatologists for escalating arthritis treatment.
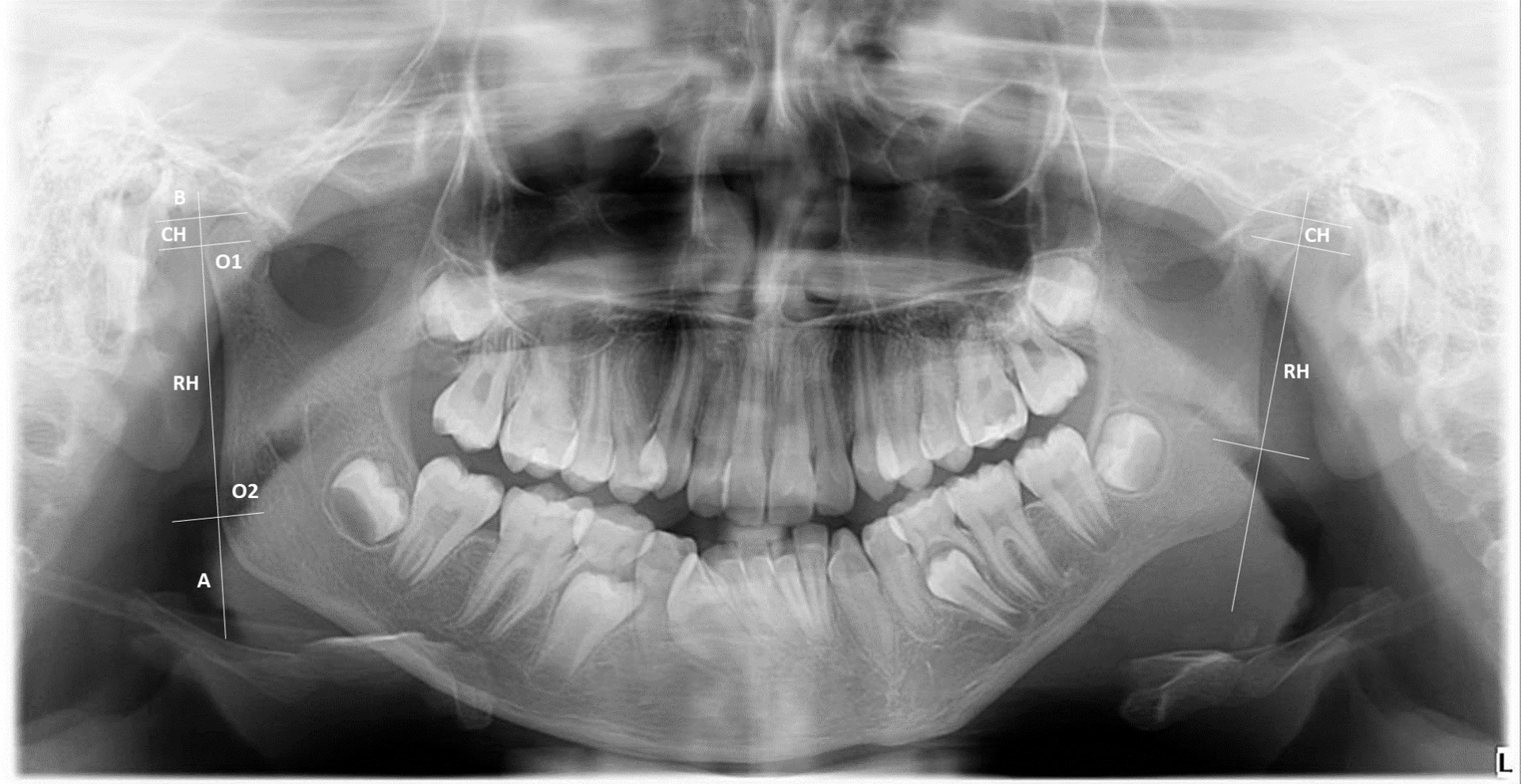
Orthopantomography (OPT) of a 12 years old girl with juvenile idiopathic arthritis (JIA) (personal archive). A: ramus tangent; O1: most lateral points of the condylar profile; O2: most lateral points of the ascending ramus profile; B: perpendicular line to line A, starting from the most superior point of the condylar profile; CH: condylar height; RH: ramus height. The absolute difference between the measured condylar or rami sizes of the right (R) and left (L) are divided by the sum of the same condylar or rami sizes (expressed in percentages, respectively). This ratio can range from complete symmetry (0%) to maximal asymmetry (100%). Equal or above the level of 6% difference between the condylar vertical sizes is considered the clinically meaningful limit for diagnosing a condylar asymmetry
Computed tomography and cone-beam computed tomography
Compared to cephalograms and OPT, computed tomography (CT) can image much more detailed bony structures. However, as with the above-mentioned conventional radiological techniques, features of active TMJ synovitis cannot be visualized by CT. Further, CT is associated with relatively high radiation exposure of patients and is not easily achievable in low-income geographical areas [41, 42].
In the recent decades, cone beam CT (CB-CT) revealed to be accurate in the visualization of bony profiles of the TMJ, with lower radiation dose compared to OPT or classic CT. Reconstruction of high-resolution 3D image provides high-quality visualization of osseous lesions, with no sensitivity for soft tissue structures. Hence, its use is limited to the assessment of structural damage following chronic inflammation and it is not suited for the detection of early signs of active TMJ synovitis [38].
Magnetic resonance imaging
Up to date, contrast-enhanced MRI (CE-MRI) is considered the gold standard for the complete assessment of TMJs in JIA, because of the large view of the joint and the possibility of extensive evaluation also of bones and soft tissues around the joint (Figure 4).
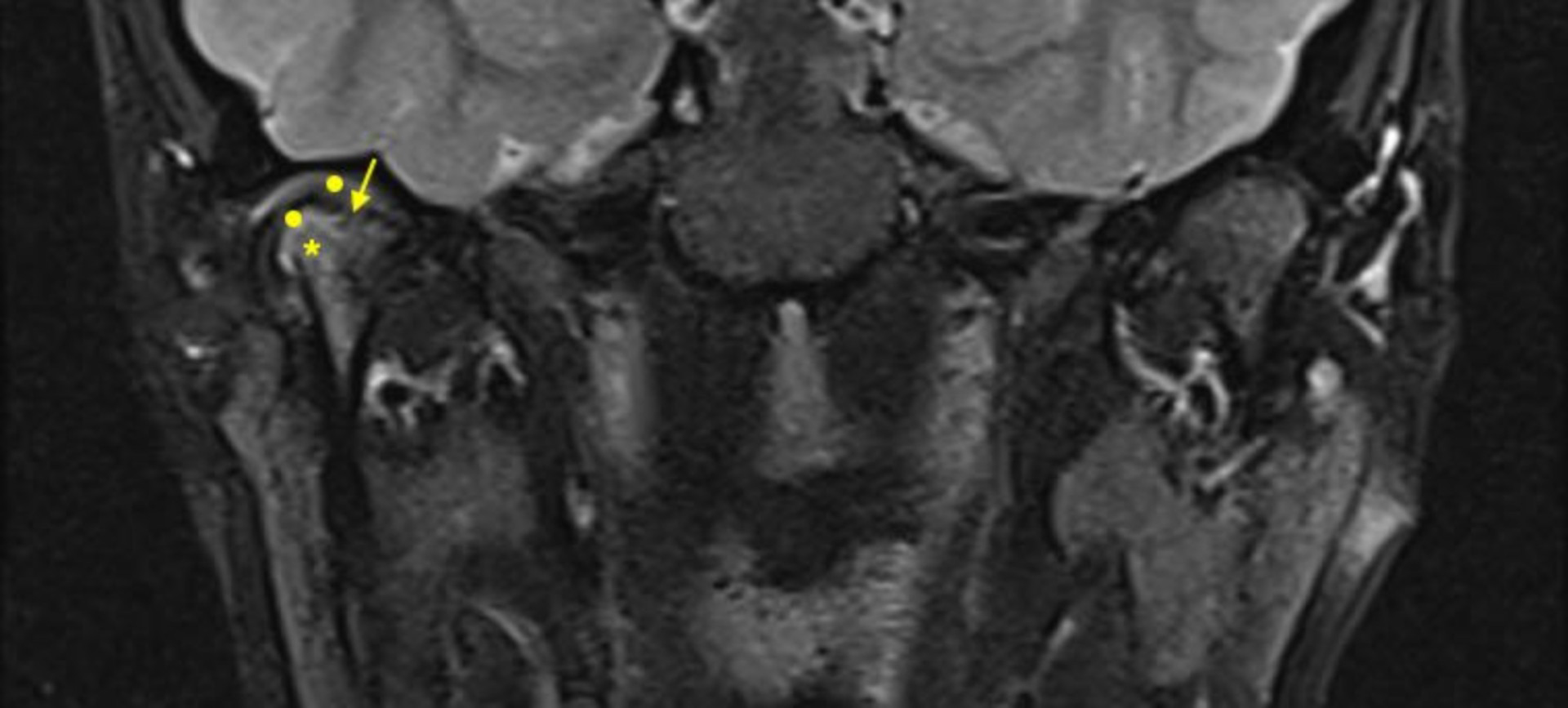
CE-MRI of a 16 years old boy with JIA. Image of unilateral (right) TMJ arthritis with associated structural alterations visualized on T2-TSE fat sat coronal sequence after contrast. Round dots: mild intermediate signal indicating synovial thickening; star: hypersignal indicating bone marrow oedema; arrow: flattened surface and irregularities of the mandibular condyle. CE-MRI: contrast-enhanced magnetic resonance imaging; JIA: juvenile idiopathic arthritis; TMJ: temporomandibular joint
CE-MRI of TMJs may require a long time of image acquisition (up to 45–60 minutes), thus limiting its use in children unless under general sedation in the less collaborative ones. To address the urgent need for short imaging protocols without compromising the accuracy of the technique, international consensus initiatives identified a minimum and optimal set of MRI sequences useful to provide accurate information on clinically relevant questions. Besides acquisition protocols, consistent work on the correct interpretation and scoring system has also been performed, with the aim of improving discriminative capacity to detect differences between patients and within-patient interval variations. In recent years, independent groups of experts followed consensus-based and data-driven selection processes, with the achievement of TMJ CE-MRI scoring systems with some differences in their compositions, grading thresholds, and imaging requirements [43–47].
For instance, the EuroTMjoint research group defined a progressive scoring system which takes into account an inflammatory domain and a bone deformity domain, and counts each item of the domains for each TMJ: synovial inflammation can be visualized as a hyper signal finding, corresponding to synovial fluid and bone marrow oedema, and as an intermediate signal finding, in case of synovial thickening and pannus formation, and can be scored as absent, mild, moderate, and severe; bone deformities include changes in the condyle, joint cavity and eminence, and temporal bone surface which can be visualized on MRI PD and T1 images and can be categorized as normal morphology or mild, moderate, severe flattening, or TMJ destruction, this further detailed as the presence of erosions and/or fragmentation [43].
Another multi-institutional study evaluated the reliability of existing systems according to the Outcome Measures in Rheumatology Clinical Trials (OMERACT) MRI guidelines. The OMERACT MRI TMJ study group proposed a semi-quantitative and additive scoring system, again considering two domains: the inflammatory domain, which includes bone marrow oedema, joint effusion, and synovial membrane thickening; and the structural domain, comprehensive of condylar flattening, erosions, and disc abnormalities. Each TMJ is evaluated and scored independently for each item; the total score is the sum of inflammatory and structural items in the two TMJ [44].
The EuroTMjoint and the OMERACT TMJ MRI scoring systems showed similar performances when applied to the same cohort of JIA patients and age-matched healthy controls, suggesting that both can be used similarly in the evaluation of TMJ MRI findings in JIA patients [48]. To further drive in the correct acquisition and interpretation of MRI images of TMJ in children, an extensive atlas of normal anatomy and normal variants of TMJs in children and in JIA patients has been recently published [49]. However, despite this valuable support, mild findings in the soft and osteochondral tissue components can still be commonly seen on TMJ CE-MRI in non-rheumatologic patients, thus limiting an ideal straightforward interpretation [50]. Indeed, studies on the discriminative ability and responsiveness of TMJ CE-MRI are hampered by the lack of external measures. Further, the minimal clinically important difference in TMJ CE-MRI findings is difficult to determine, since the relevance of subclinical findings should be demonstrated before a clinical impact occurs. In addition, it must be acknowledged that TMJ MRI is not widely available, it is costly, and it requires intravenous contrast sequences and often sedation in children. Hence, it can be performed in selected cases and cannot be recommended for routine screening or short-time interval assessments in JIA patients.
Ultrasound
In the past two decades, musculoskeletal ultrasound (MSUS) has been increasingly used in routine clinical practice for the evaluation of a large number of joints in children with JIA [51]. Its usefulness for the TMJs has been seldom investigated due to the complex anatomy of the joint and its limited view with this imaging technique, since only the lateral aspect of the joint can be seen. As consequence, there are no accepted MSUS-based normative values for synovial thickness neither any scoring system specifically for the TMJ.
A recent systematic literature review highlighted the disomogeneity of TMJ MSUS features and of methodologies applied in the publications, which make it difficult to compare results. Nonetheless, MSUS was revealed to be not informative on joint effusion and synovial vascularization through power Doppler signal, whereas synovial thickening is detectable, but with no distinction between active or inactive TMJ synovitis. Condylar deformities, in particular remodeling, but also surface irregularity, erosion, flattening, osteophytes, and abnormalities at the condylar disk can be visualized by TMJ MSUS, although to a lesser extent when compared to TMJ MRI [52].
Notably, some authors used the lateral periarticular joint space (LPAS) measured from the condyle’s cortical contour to the capsule’s contour as indirect parameters of the synovial joint space and found statistically significant differences between JIA patients and controls (0.086 cm and 0.055 cm, respectively; p = 0.000). In addition, LPAS measured with US and MRI showed a positive correlation (Spearman test: p of 0.623 and p < 0.05) [53]. These results suggest LPAS as a potential sonographic parameter of the synovial thickness of TMJ in JIA patients, which could be useful in the detection of early TMJ alterations (Figure 5).
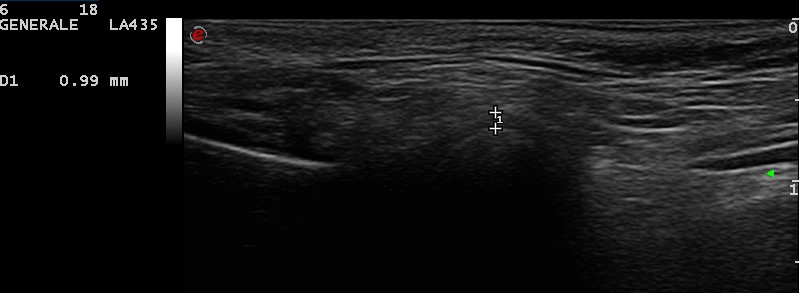
Lateral periarticular joint space (LPAS) measured from the condyle’s cortical contour to the capsule’s contour in a 4 years old female child (personal archive). The symbols “+” on the figure indicate the capsule’s contour (upper +) and the condyle’s cortical contour (lower +); “1” represents the distance between the two reference points. As outlined in the output of ultrasound screen (left side), 1 (i.e., LPAS) measures 0.99 mm
Discussion
The present perspective highlights that several international multidisciplinary initiatives provided their multifaceted efforts in early detecting TMJ arthritis in patients affected by JIA. Considering the potentially severe complications, timely diagnosis and appropriate treatment of TMJ involvement in JIA are of great importance. Currently, pharmacological treatment options for TMJ arthritis include intraarticular glucocorticoid injections (IAGI), conventional disease-modifying drugs (c-DMARDs), or biologic therapies (b-DMARDs) [54]. Physiotherapy, occlusal and distraction splints, and functional orthodontic appliances are sometimes adopted, largely depending on the interaction among the specialists and the availability of services to families [55]. Of note, TMJ IAGI are not recommended in pre-pubertal ages, whereas c-DMARDs and b-DMARDs may be indicated for concomitant involvement of other joints, independently of TMJ. Hence, in JIA patients solely treated with IAGI for oligoarthritis in joints other than TMJ or in those on treatment with c-DMARD and/or b-DMARD, TMJ arthritis may be under-investigated, and hence under-diagnosed and potentially under-treated, for both inflammatory and structural features [56, 57].
The orofacial examination has still an important role as screening for supporting indication to further investigations, which cannot be thoroughly applied for the above-mentioned limitations. The recommendation of a routine standardized orofacial examination approach is now further enhanced by the availability of consensus-based JIA-specific orofacial examination protocol, recently published [34].
Conventional radiography and CB-CT are employed to analyze condylar bone abnormalities and offer excellent resolution of cortical surfaces. Both cannot sufficiently analyze soft tissue and are associated with radiation exposure. Therefore, their use is limited to long-term monitoring of structural alterations [58, 59]. On the other side, CB-CT is considered the gold standard for assessing bony TMJ components, i.e., TMJ involvement in JIA, particularly useful when CE-MRI cannot be performed [24, 30]. OPT may still play a role in screening children with JIA, particularly at younger ages for the selection of those deserving of MRI examination.
Hitherto, CE-MRI is considered the gold standard for the complete assessment of TMJs in JIA, because of the large view of the joint and the possibility of extensive evaluation also of bones and soft tissues around the joint. Nonetheless, TMJ CE-MRI still carries some challenges in its metric properties and its findings need to be cautiously interpreted. Moreover, it is not widely available, it is costly, and it requires intravenous contrast sequences and often sedation in children. Hence, TMJ CE-MRI cannot be recommended for screening in JIA patients and can be used in selected cases.
Despite the recent dramatic advances in the applicability of MSUS in JIA [60], this imaging technique has been poorly used for TMJ assessment. The narrow acoustic window and the capability to visualize only a small superficial part of the joint represent intrinsic limitations. In addition, the lack of a standardized image acquisition technique in TMJ widens the operator dependency. Therefore, the interpretation of TMJ sonographic findings in JIA patients is controversial. Nonetheless, due to the recent technical advances in MSUS machines, the increasing availability of high-frequency probes that allow more clear and precise images of superficial structures, the reduction in the costs of US machines, and the spreading of MSUS in pediatric rheumatology, MSUS may be further explored for the assessment of TMJ in JIA [52, 61]. LPAS measured by MSUS is a candidate feasible measure of the synovial thickness of TMJ in JIA patients for early identification of TMJ arthritis.
Conclusions
Due to the anatomical and functional complexity of TMJs and the physiological changes that occur during growth, timely diagnosis of TMJ arthritis in JIA represents a challenge. Advances in imaging modalities and multidisciplinary international efforts are efficiently contributing to solving the limitations of each approach. Further investigations need to be kept in the next agenda for prompt, reliable, safe, and affordable screening of TMJ involvement in JIA patients. Since TMJ disease may occur initially in one joint and involve the other over time, standardization in the detection of TMJ arthritis would also allow us to better capture and understand the natural history of this joint involvement throughout the JIA disease course.
Highlights and directions
The TMJaw short screening protocol is a quick TMJ assessment in JIA patients, applicable by different specialists in the routine clinical setting. Prospective studies would allow investigation of its prognostic value in the early detection of TMJ involvement in JIA.
OPT is characterized by low radiation exposure and wide availability in dental offices; despite it provides no information on inflammatory alterations, early identification of changes in the asymmetry index may support the selection of JIA patients toward timely advanced investigations.
Though it is considered the current “gold-standard” in the assessment of TMJ arthritis, TMJ CE-MRI still carries some challenges in its metric properties. Therefore TMJ CE-MRI findings need to be cautiously interpreted. Studies on its discriminative ability and responsiveness are hampered by the lack of external measures. The minimal clinically important difference in TMJ CE-MRI findings has not been determined yet. Further development in MRI technique would maybe allow for examination without intravenous contrast agents and shorter acquisition protocols, thus enabling a wider application in younger children with JIA.
The LPAS of the TMJ in JIA measured by MSUS proved to be correlated with LPAS measured by MRI. It may represent a quickly available measure of the synovial thickness of TMJ in JIA patients. Its potential usefulness in the detection of early TMJ alterations should be further investigated.
Abbreviations
| b-DMARDs: | biologic disease-modifying drugs |
| CB-CT: | cone beam computed tomography |
| c-DMARDs: | conventional disease-modifying drugs |
| CE-MRI: | contrast-enhanced magnetic resonance imaging |
| IAGI: | intraarticular glucocorticoid injections |
| ILAR: | International League of Associations for Rheumatology |
| JIA: | juvenile idiopathic arthritis |
| LPAS: | lateral periarticular joint space |
| MSUS: | musculoskeletal ultrasound |
| OMERACT: | Outcome Measures in Rheumatology Clinical Trials |
| OPT: | orthopantomography |
| RF: | rheumatoid factor |
| TMJ: | temporomandibular joint |
| TMJaw: | Temporomandibular Joint Juvenile Arthritis Working Group |
| US: | ultrasound |
Declarations
Acknowledgments
The author thanks Dr. Lorenzo Maria Gregori and Dr. Giulia Vallogini for the insights provided during the clinical care of JIA patients, Marina Olmo for language revision.
Author contributions
SMM: Conceptualization, Investigation, Writing—original draft, Writing—review & editing.
Conflicts of interest
The author declares no conflicts of interest.
Ethical approval
According to the ICMJE guidelines and local rules, no ethical approval was required for the current manuscript. Patients’ involvement complied with the Declaration of Helsinki.
Consent to participate
Informed consent to participate in the study was obtained from all participants or legal guardians for children under 16.
Consent to publication
Informed consent to publication was obtained from all relevant participants or legal guardians for children under 16.
Availability of data and materials
All datasets for this study are included in the manuscript.
Funding
Not applicable.
Copyright
© The Author(s) 2025.
Publisher’s note
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.