Affiliation:
1Division of Rheumatology, Allergy, and Immunology, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA
Email: jyinh@mgh.harvard.edu
ORCID: https://orcid.org/0000-0002-6390-0941
Affiliation:
2Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, USA
ORCID: https://orcid.org/0000-0002-8095-0174
Affiliation:
3Department of Plastic Surgery-Orthopedic Surgery, New England Baptist Hospital, Tufts School of Medicine, Boston, MA 02120, USA
ORCID: https://orcid.org/0009-0001-5559-8631
Affiliation:
4Department of Radiology, Boston Healthcare System, Boston University School of Medicine, Boston, MA 02132, USA
ORCID: https://orcid.org/0000-0002-9374-8266
Explor Musculoskeletal Dis. 2025;3:100786 DOI: https://doi.org/10.37349/emd.2025.100786
Received: October 31, 2024 Accepted: January 04, 2025 Published: February 24, 2025
Academic Editor: Peter Mandl, Medical University of Vienna (MUW), Austria
The article belongs to the special issue Multifaceted Imaging in Rheumatic and Musculoskeletal Diseases
Calcium pyrophosphate deposition (CPPD) disease is the most prevalent crystal related arthropathy in the older-aged population. The clinical spectrum of CPPD arthropathy can include asymptomatic, acute, and chronic inflammatory disease that primarily results in irreversible structural joint damage. The manifestations and impact of structural damage that result from other inflammatory non-degenerative features of chronic CPP crystal inflammatory arthritis are often underrecognized and may lead to an inaccurate perception of disease severity. This pictorial essay sought to display the disease burden and various presentations of chronic inflammatory CPP crystal arthritis. Sonographic imaging of cases with chronic CPP arthritis was presented for diagnostic considerations and to demonstrate the extent of irreversible articular and peri-articular damage occurring at a structural level. Evolving understanding of the imaging characteristics of CPPD disease will provide a better appreciation of disease morbidity caused by this condition and stress the need to expand efforts to find effective treatments to dissolve crystal deposition and targeted therapies that will contain the inflammatory response to crystals. Recognizing the anatomic consequences of tissue damage that arise from CPPD disease should also help reflect on potential surgical alternatives to mitigate the progression of structural complications until effective disease modifying agents for this condition emerge.
Calcium pyrophosphate deposition (CPPD) disease is an inflammatory arthropathy predominantly seen in older individuals that is a consequence of an exaggerated inflammatory response to aberrant CPP crystal formation and deposition within the cartilage [1]. This clinically heterogeneous condition has varied presentations: asymptomatic CPPD, acute CPP arthritis (pseudogout), chronic CPP arthritis manifesting as persistent inflammatory arthritis, CPPD with osteoarthritis (OA), and crowned dens syndrome [2]. Chronic CPP inflammatory arthritis leads to cartilage degradation, subchondral bone changes, and osteophyte formation that culminate in end-stage degenerative joint disease. In contrast to early CPPD disease classification criteria described by McCarty [3], updated 2023 criteria by Abhishek et al. [4] are more inclusive of these clinical presentations and recognize the role of other non-radiographic imaging modalities to better classify a patient as having CPPD disease.
Chronic CPP arthritis is an underrecognized variant of CPPD disease that can overlap and/or be misdiagnosed as OA, polymyalgia rheumatica (PMR), or rheumatoid arthritis (RA). The true prevalence of chronic presentations of CPP arthritis has not been well defined [5], however, studies characterizing the radiographic and clinical phenotypes of CPPD disease in patients with early diagnosis of seronegative RA have reported a prevalence of 3.9% of CPPD disease in seronegative RA, with a percentage of 7% among patients > 60 years at baseline [6]. Earlier descriptions of patterns of joint involvement in CPPD disease estimate a frequency of chronic polyarticular degenerative CPP arthritis in up to 50% of CPPD disease cases [2]. Chronic OA-like or degenerative CPPD disease with episodic flares and unusually severe articular destruction can be seen as atypical OA joint distribution involving the glenohumeral (GH), wrist, metacarpophalangeal (MCP) [2], and scaphotrapeziotrapezoid (STT) joints [7].
The chronic CPP inflammatory phenotype may manifest as a mono- or polyarticular disease. In the latter scenario, symmetric involvement of the hands and wrists is a mimicker of RA, hence the term “pseudo-RA” [6]. The clinical resemblance of chronic CPP crystal inflammatory arthritis with seronegative RA may at times make these conditions clinically indistinguishable. This is further compounded by restrictions in consistently obtaining synovial fluid to confirm the CPPD of cases in question and the limitations of synovial fluid analysis in accurately identifying CPP crystals under polarized microscopy [8]. Descriptions of misdiagnosed chronic inflammatory CPP arthritis in patients diagnosed with early seronegative RA should create awareness of the need to reconsider CPPD disease in cases of seronegative RA presenting in the elderly with suitable clinical picture of crystal arthritis and evidence of CPPD on imaging [6]. This is further supported by findings of higher prevalence of chondrocalcinosis (CC) and CPPD co-morbidity in seronegative (32.3%) vs. seropositive (16.6%) RA patients, the former also found to be typically older patients at diagnosis and more frequently present with acute attacks [9]. The musculoskeletal morbidity of chronic CPP arthritis has not been described as it may be concealed by what is thought to be seronegative RA. Lack of effective therapies to dissolve CPP deposits or disease modifying agents in CPPD disease are major determinants that are contributing to severe musculoskeletal structural damage as previously witnessed in the past in RA in the pre-biologic era.
Updated classification criteria for CPPD disease have adopted a similar approach to classification criteria for gout, where ultrasound (US) and dual-energy CT are weighted equally to gold standard synovial crystal analysis [10]. This has been reflected in the incorporation of CPPD imaging definitions of advanced imaging modalities in the preparation of the recently published CPPD classification criteria [11]. Among the imaging modalities found useful in the assessment of CPPD disease, the US has the multi-faceted advantage of showing structural derangement, concurrent surrounding soft tissue inflammatory damage, and clear identification of calcific deposition in various tissues [12]. In addition, the US has been proven to be as accurate as synovial fluid analysis in the diagnosis of CPPD disease, with a reported sensitivity of 96% and specificity of 87% [13].
Asymptomatic CPPD has been defined as CPPD with no apparent clinical consequence, which can be isolated CC or OA with CC [5]. The notion of CPPD being asymptomatic has been questioned given the known deleterious effects of CPPD in cartilage and evidence on cross-sectional studies demonstrating an association between CC with increased radiographic severity [14], pain [15], and disability in knee OA [16]. Nonetheless, confirmation of CPPD igniting or universally leading to disease has not been proven to date, and there are contradicting epidemiologic studies showing the presence of CC in symptomatic knee OA not affecting the risk of arthroplasty, disease progression [17], or cartilage loss [18].
The interplay between crystal related arthritis and OA is an area that has perturbed experts in the field and a topic that has been recognized as a priority to investigate in the pathogenesis of CPPD disease [19]. Most of the efforts to understand the pathogenic role of CPPD in the development of OA have primarily addressed the characteristics of knee OA with and without CPPD [14–18]. It may be more informative to seek these answers in the OA of other joints like the shoulder or wrist, which are typically not expected to present with primary OA as seen in the knee which is also a prevalent joint developing primary OA associated with many confounders. Prospective studies assessing the association of CPPD with OA may likely favor the speculated causality of CPPD in OA if longer follow-up periods beyond 5 to 10 years are undertaken [20, 21] (Figure 1A and B). In addition, the CPPD disease burden involving periarticular soft tissues that are mainly identified through cross-sectional imaging (such as CT, magnetic resonance imaging, or US) is less well evaluated using conventional radiographs. Moreover, the role of other imaging tools in assessing the extent and consequences of periarticular damage in CPPD has not been well described (Figure 1C–G). Other factors that remain to be elucidated are the clinical or genetic factors that drive disease in the subset of cases where CC progresses to disease and identifying markers of disease activity.
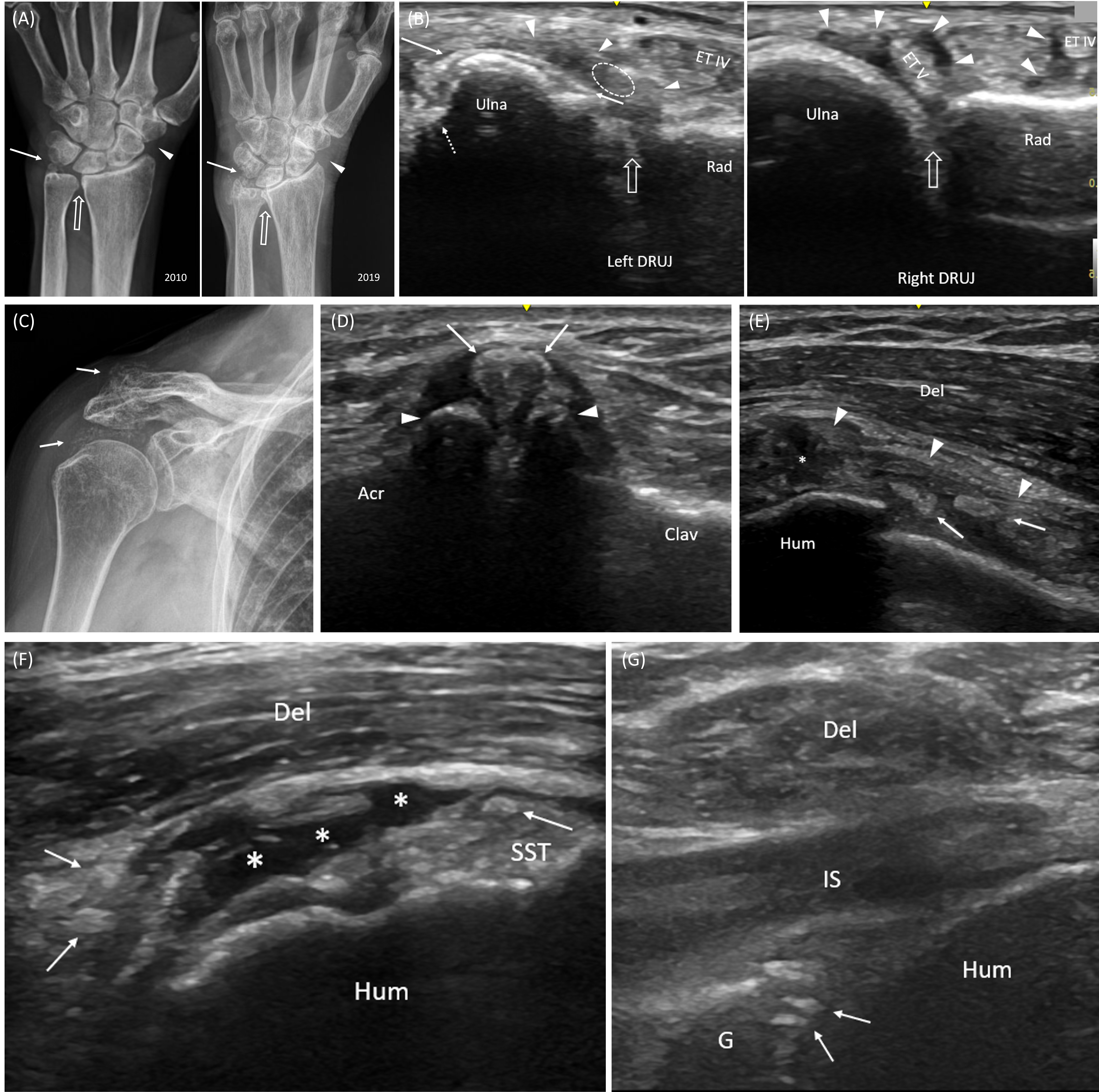
Chronic soft tissue inflammation in CPPD disease related to irreversible structure damage. Example 1: progression of articular and soft tissue damage in CPPD disease of the wrist. (A) Wrist radiographs show soft tissue swelling and CPPD at the triangular fibrocartilage (white arrow) with early degenerative disease of the STT joint (white arrowhead) interpreted clinically as STT OA and overlapping chondrocalcinosis. Note preserved distal radioulnar joint (hollow arrow). Wrist radiograph after a 9-year progression demonstrates denser CPPD at the triangular fibrocartilage (white arrow), evolving degenerative changes of the carpometacarpal and STT joint (white arrowhead), and the development of distal radioulnar (hollow arrow) joint OA; that are not usual locations expected to develop primary OA. (B) Corresponding transverse ultrasound image of the proximal left wrist shows synovitis (white arrowheads) with secondary OA changes of the distal radioulnar joint with infiltrating synovium occupying the space of the absent 5th extensor tendon (extensor digiti minimi) that has spontaneously ruptured (dotted oval). There is also cortical disruption of the distal radioulnar joint (hollow arrow) with distortion of the round bony cortex of the ulna that has a hyperechoic thin CPPD over the ulnar cartilage (white arrow) and denser CPP rounded hyperechoic deposits with early erosive changes at the ulnar groove (dotted white arrow). The contralateral right wrist shows preserved distal radioulnar joint (hollow arrow) and preserved bony contours of the ulna and radius. There is hypoechoic tenosynovitis surrounding the 4th and 5th extensor compartments (white arrowheads). Example 2: representation of the extent of periarticular structural damage in CPPD disease of the shoulder identified on US that is not captured on conventional radiograph. (C) Shoulder radiograph showing extensive soft tissue mineralization overlying the acromioclavicular joint and in the region of the rotator cuff and/or subacromial-subdeltoid bursa (white arrows) with moderate acromioclavicular and mild glenohumeral degenerative changes: case that can be technically categorized as ‘OA of the acromioclavicular joint with CPPD’. (D) US evaluation of the shoulder structures demonstrating the degree of periarticular structural damage related to CPPD not perceivable on plain radiographs. Long axis view of the acromioclavicular joint demonstrates large osteophytes (white arrowheads) with extrusion of the calcified fibrocartilage (white arrows) and capsular distention. (E) Anterior long axis view of the biceps tendon shows absent ruptured (asterisk) and retracted biceps tendon space replaced by oval shaped hyperechoic CPP deposits (white arrows) and thickened hypoechoic overlying subacromial-subdeltoid bursa (white arrowheads). (F) Long axis view of the supraspinatus tendon demonstrates severe tendinosis with a bursal surface tear (asterisks) and numerous calcific deposits within the tendon body and attachment (white arrows). (G) Posterior short axis view of the glenohumeral joint shows mild synovial proliferation of the joint and CPPD within the labrum (white arrows). Acr: acromion; Clav: clavicle; CPP: calcium pyrophosphate; CPPD: calcium pyrophosphate deposition; Del: deltoid; DRUJ: distal radioulnar joint; ET: extensor tendon; G: glenoid; Hum: humerus; IS: infraspinatus tendon; OA: osteoarthritis; Rad: radius; SST: supraspinatus tendon; STT: scaphotrapeziotrapezoid; US: ultrasound
Erosions that occur as a consequence of chronic synovitis are traditionally lesions associated with RA and gout. Although CPPD disease is not typically categorized as an erosive arthropathy, patients with persistent inflammatory chronic CPP disease have ongoing synovitis that progresses to erosive changes and joint dysfunction of similar severity to that seen in uncontrolled gout and RA. A prospective case-control study assessing the MCP joints of patients with CPPD vs. RA reported the presence of erosions in a minority of patients with CPPD disease with a frequency of 4.8% vs. 50% in RA (p < 0.01). The authors concluded that the presence of erosions was the US finding of the highest value to diagnose RA [22]. A cross-sectional retrospective study including over 250 patients with the diagnosis of CPPD or RA found the presence of erosions nearly equally prevalent in seronegative RA (27%) and cases of CPPD (25%); with an expectedly higher prevalence of erosions (44%) in patients with seropositive RA (p 0.001) [9]. Alternatively, a series of 435 patients evaluating the prevalence of CC in seronegative RA found 3.9% of cases to have overlapping CPPD disease, with none of these patients developing ‘typical RA-like erosions’ over a 10-year follow-up period [6]. Coexisting CPPD and gout with erosive polyarticular disease have also been described [23].
Erosions found in cases of CPPD disease may be diagnostically misleading. Erosive changes in CPPD are generally noted to occur in areas of denser CPPD with surrounding inflammation like the ulnocarpal joint, triangular fibrocartilage complex (TFCC), radiocarpal, and MCP joints; which are also areas commonly affected in RA (Figure 2A–F). Furthermore, CPPD disease with erosions can be found involving the 1st MTP (metatarsophalangeal) which is more frequently a site of gouty arthropathy (Figure 2G and H). Evidence on imaging of dense CPPD with synovitis adjacent to erosions would strongly support calcific crystal related disease as the most likely condition leading to erosions. The presence of CPPD crystals surrounded by giant cells within the synovium reflective of what is seen in the US has been evidenced on histopathologic specimens of tissue extracted from cases of CPPD after surgical intervention for tendon ruptures and carpal tunnel decompression in CPPD disease [24].
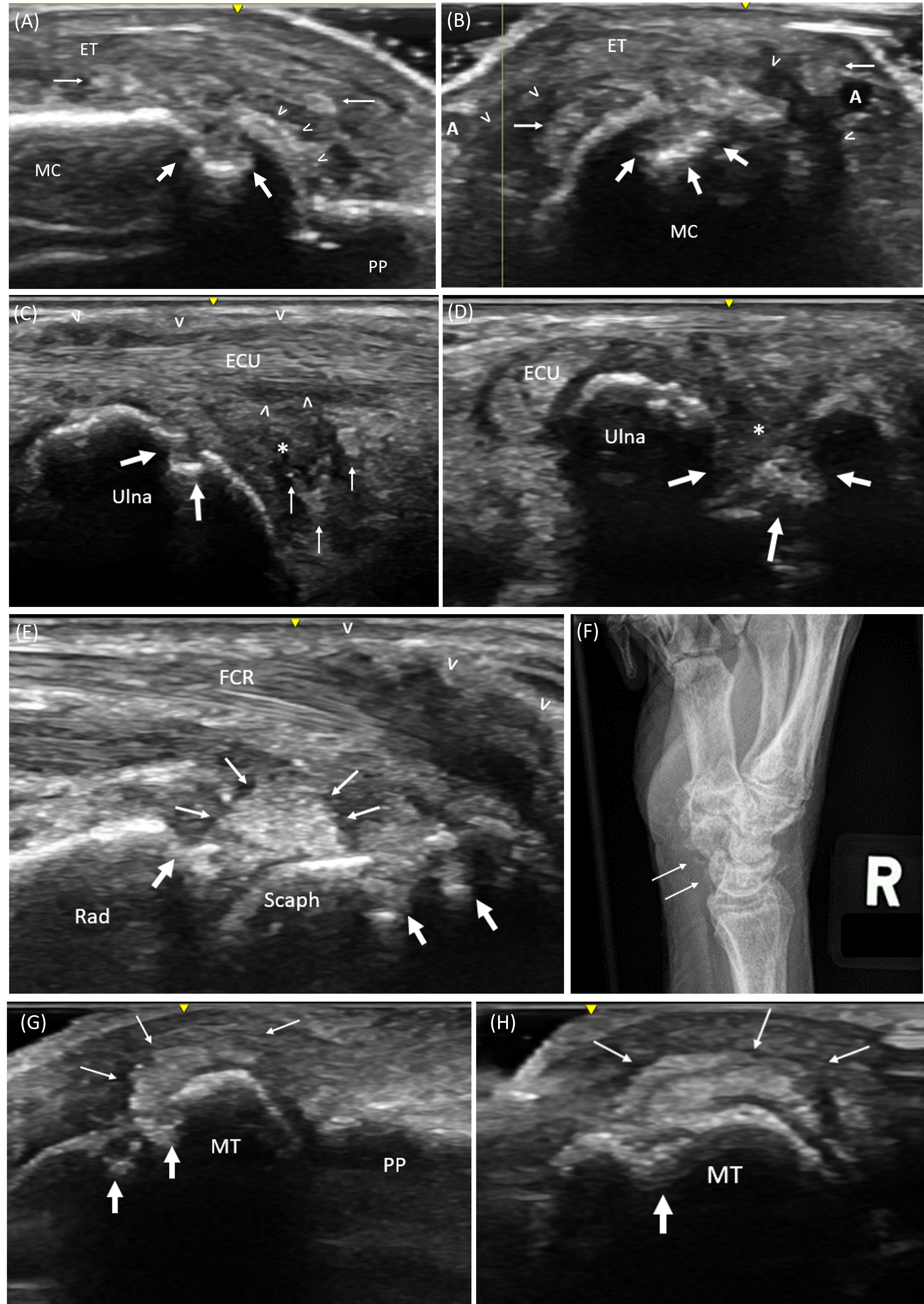
Erosions at various locations in chronic inflammatory CPPD disease. Example 1: metacarpal 2 erosion and chondrocalcinosis. (A) Long axis sonographic view of the 2nd MCP joint shows large erosion of the metacarpal head (thick white arrows) with a thin hyperechoic rim of CPP cartilage deposition (open arrowheads) and larger nodular hyperechoic CPP deposits (thin white arrows). There is a narrowing of the joint space, surrounding synovitis, and tendinosis of the overlying extensor tendon. (B) Short axis view of the metacarpal head confirming erosion (thick white arrows) with surrounding CPPD (thin white arrows) and synovitis (open arrowheads). Example 2: ulnar erosion and CC of the TFCC. (C) Long short axis view of the ulnar wrist showing synovitis of the ulnocarpal joint (asterisk), intra-articular calcific deposition (thin white arrows), ulnar erosion (thick white arrows), and tenosynovitis of the extensor carpi ulnaris tendon (open arrowheads). (D) Short axis view also shows an eroded ulnar groove (white arrows) and subluxation of the ECU from its normal position (asterisk). Example 3: erosions of the scaphoid and CPPD. (E) Volar longitudinal view of the wrist at the level of the FCR tendon shows tenosynovitis (open arrowheads), synovitis with large hyperechoic calcific deposition within the synovium (thin white arrows) over the area of erosions (thick white arrows) of the distal radius and scaphoid bone. (F) Corresponding wrist radiograph shows mineralization (white arrows) at the volar radiocarpal joint but inflammatory features and erosive changes are not visible. Example 4: metatarsophalangeal erosion and CPPD. (G) Longitudinal and (H) transverse view of the medial aspect of the 1st MTP showing erosions (thick white arrows) that are deep to the area of hypoechoic synovium containing large hyperechoic nodular signal from calcific deposition (thin white arrows). Cases confirmed to have CPP crystals on synovial fluid analysis. A: artery; CPP: calcium pyrophosphate; CPPD: calcium pyrophosphate deposition; ECU: extensor carpi ulnaris tendon; ET: extensor tendon; FCR: flexor carpi radialis; MC: metacarpus; MTP: metatarsophalangeal; PP: proximal phalanx; Rad: radius; Scaph: scaphoid; TFCC: triangular fibrocartilage complex
Chronic tenosynovitis is a relatively common feature of chronic CPP inflammatory arthritis that is underrecognized and scantly reported in the literature [25, 26]. Most of the literature pertaining to US findings in CPPD has focused on its role in diagnosing CPPD in various tissues including the tendon and ligaments [22, 27], but there is limited mention of other associated inflammatory features encountered in CPPD disease. In the lead author’s experience, chronic recalcitrant tenosynovitis is not infrequently seen in the extensor tendons of the wrist (Figure 3A–C), flexor tendons of the fingers (Figure 3D), ligaments of the wrist (Figure 3E–H) and less frequently, in the shoulder tendons (Figure 1E and F). Spontaneous ruptures of tendons of the wrist reported at a prevalence of 4% in chronic RA patients [28], is a feature that has been reported in a number of cases associated with CPPD disease [24, 25, 29]. Presumably, CPPD within the tendon leads to damage similar to what is noted in the cartilage, and chronic surrounding inflammation consequent to CPPD leads to tendon fragility and subsequent rupture in the absence of significant trauma. Other factors reported to possibly contribute to spontaneous tendinous ruptures in CPPD include tophaceous ‘pyrophosphate’ deposition of the tendon [30], synovial proliferation, and dorsal prominence of the ulnar head [25]. Chronic CPP crystal inflammatory arthritis may also present as persistent tenosynovitis of the flexor tendons that can present as stenosing tenosynovitis that is recalcitrant or affects multiple digits, dactylitis appearing digits [31], and in chronic cases, consequent A2 pulley tear may result from adjacent tenosynovitis of the flexor tendon (Figure 3D).
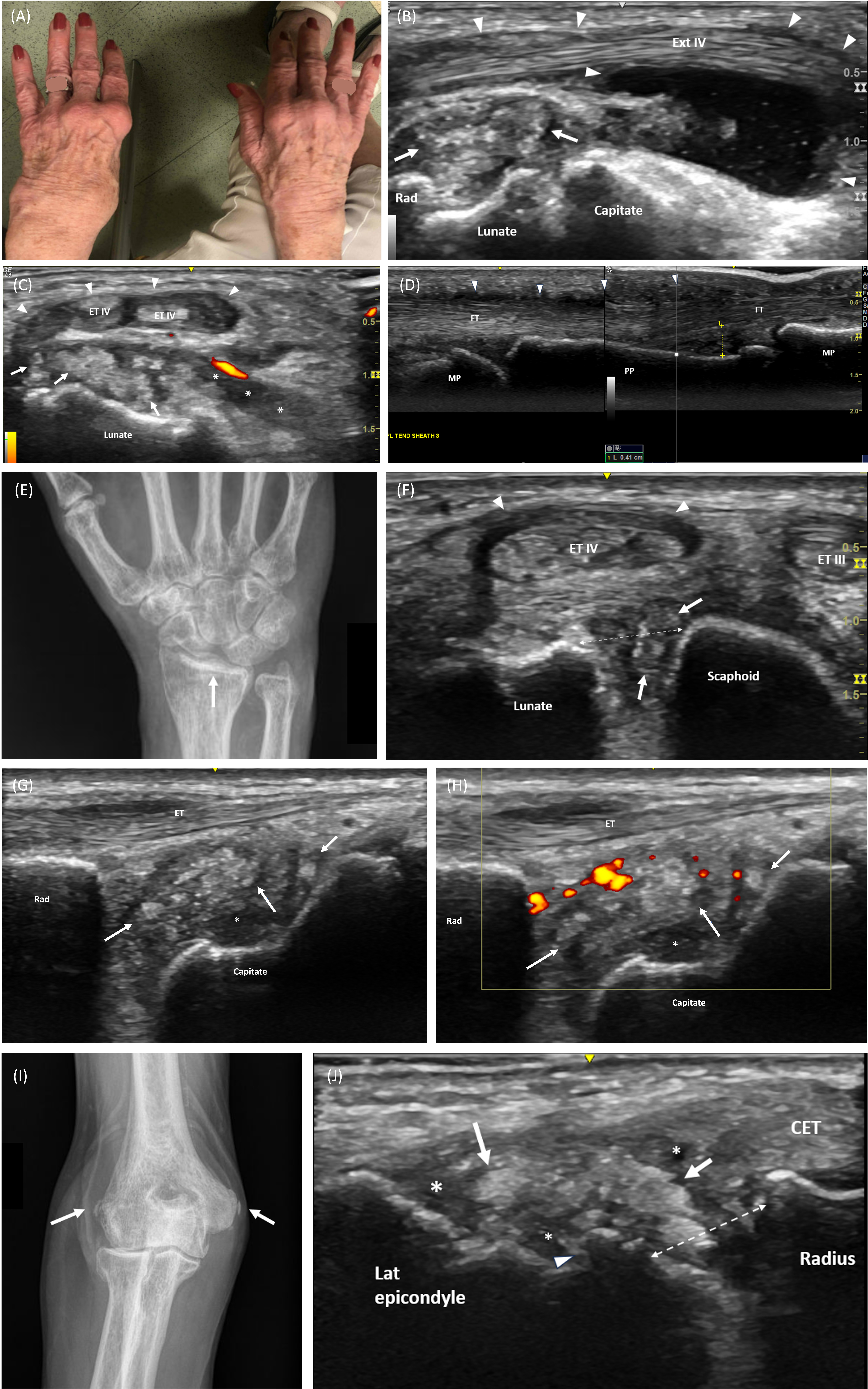
Tendon and ligamaent damage in CPPD disease. Example 1: chronic tenosynovitis of the extensor tendons of the wrist. Chronic CPP arthritis in a 79-year-old patient presenting with chronic arthritis of the left wrist with extensor tenosynovitis and chronic synovitis of the right 2nd and 3rd MCP joints. (A) Hand photography shows marked swelling of the left wrist and the bilateral 2nd and 3rd MCP joints. (B) Long axis view of the left radiocarpal joint shows prominent tenosynovitis and fluid of the 4th extensor compartment (white arrowheads) with large globular hyperechoic CPP deposits (white arrows) over the joint. (C) Corresponding short axis view of the wrist shows synovitis (asterisks) with large intra-articular CPP deposits (white arrows) and hypoechoic tenosynovitis of the overlying 4th extensor compartment (white arrowheads). Example 2: chronic tenosynovitis of the flexor of the tendon with A2 pulley rupture. (D) Flexor tenosynovitis of the finger with A2 pulley rupture. Volar longitudinal ultrasound image of the finger of a 77-year-old male with known crystal proven CPPD disease with pain and dysfunction of the 3rd finger and no prior history of hand injury shows flexor tendinosis with tenosynovitis showing as a thick hypoechoic tendon sheath (white arrowheads). There is an abnormal gap (yellow dotted line) with resisted flexion of the finger between the tendon and the shaft of the proximal phalanx (“bowstringing” of the flexor tendon) confirming disruption of the A2 pulley. The A2 pulley that should normally be found on the surface of the tendon at the level of the proximal phalanx is not visible and obscured by tenosynovial hypoechoic thickening. Example 3: scapholunate advanced collapse in CPPD. (E) Wrist radiograph of a 69-year-old male with narrowing of the triscaphe and carpometacarpal joint. There is a widening of the scapholunate interval (white arrow) with no clear evidence of CC on the radiograph. (F) Corresponding sonographic short axis view of the wrist at the level of the scapholunate interval that shows widening (double sided white arrow) and hyperechoic CPP deposits (white arrows), the SL ligament is not visible suggesting ligament disruption. There is also tenosynovitis (white arrowheads) of the 4th extensor compartment. (G) Long axis dorsal wrist view shows synovitis (asterisk) encasing the CPPD (white arrows) likely contributing to weakening and disruption of the SL ligament and subsequent osteoarthritis (scapholunate advanced collapse, SLAC). (H) Long axis view of the dorsal radiocarpal joint showing synovitis (asterisk) with power Doppler signal and unequivocal evidence of amorphous CPP deposits (white arrows) in the gap between the scaphoid and capitate that are not appreciated on the plain radiograph. Note the absent displaced lunate that should be found distal to the radius between the lunate and capitate. CET interstitial tear and radial collateral ligament tear of the elbow in CPPD. Example 4: lateral elbow: calcific deposition and tear of the common extensor tendon and lateral collateral ligament tear. (I) Anteroposterior elbow radiograph of the elbow demonstrating ossific densities adjacent to the lateral and medial epicondyle (white arrows) as well as bony proliferative change at the radiocapitellar and ulnotrochlear joints. (J) Long axis view of the lateral elbow shows severe CET tendinosis with dense amorphous CPPD within the tendon body (white arrows) and interstitial tears of the tendon (asterisks) and cortical defect at the tendon attachment (white arrowhead). The radial collateral ligament is disrupted and not visible leading to widening and instability (double sided white arrow) of the lateral radiocapitellar joint. CC: chondrocalcinosis; CET: common extensor tendon; CPP: calcium pyrophosphate; CPPD: CPP deposition; ET: extensor tendon; Ext: extensor; FT: flexor tendon; MCP: metacarpophalangeal; MP: middle phalanx; PP: proximal phalanx; Rad: radius; SL: scapholunate
Deposition of CPP involving the scapholunate ligament ultimately resulting in ligament disruption and scapholunate advance collapse (SLAC) deformity is a condition commonly associated with CPPD disease of the wrist (Figure 3E and F) [32]. Other areas of CPPD that can lead to chronic synovitis with ligament damage and joint instability include the TFCC [32], and radial collateral ligament of the elbow (Figure 3I and J).
Chronic carpal tunnel syndrome (CTS) is a complication of CPPD disease of the wrist that has been reported to occur in approximately 36% of patients randomly sampled with wrist CC. Histologic specimens of wrists undergoing carpal release and finger flexor tenosynovectomy have demonstrated CPPD deposits isolated from the volar carpal ligament, synovium of the wrist, and finger flexor tendon sheath [33]. In chronic CPP inflammatory arthritis of the wrist, there are a number of factors contributing to a mass effect within or adjacent to the carpal tunnel leading to chronic CTS and long-term nerve compression sequelae: CPP deposits of the volar wrist (Figure 4A and B), tenosynovitis of the flexor tendons (Figure 4C and D), osteophytes of the volar carpal bones with surrounding synovitis and calcific deposition (Figure 4E–H) [7, 34].
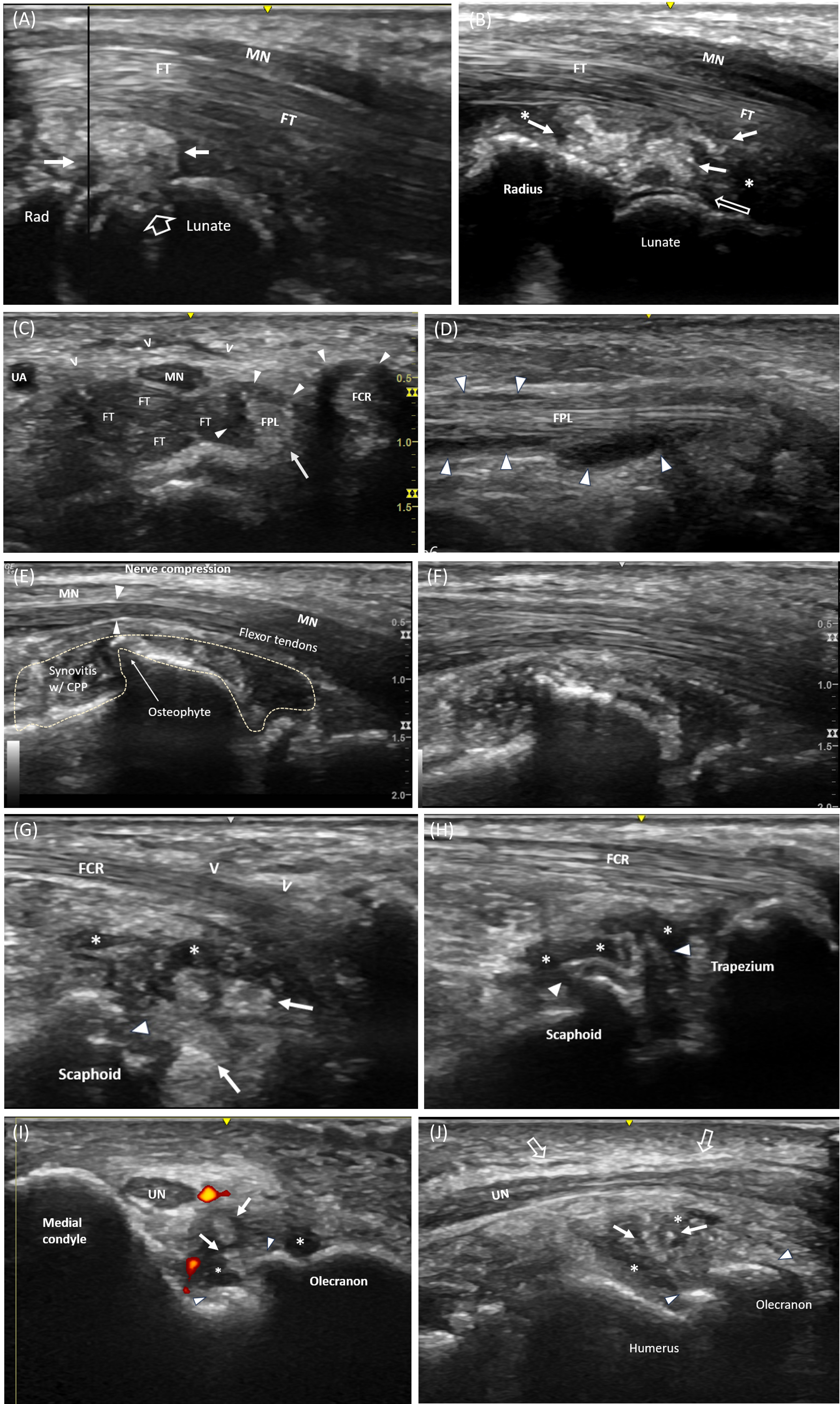
Nerve compression from chronic inflammatory CPPD disease. Example 1: two cases of crystal proven CPPD with peri-articular calcinosis and CTS. (A) Long axis view of the volar radiocarpal joint demonstrating large globular CPPD (white arrows) with adjacent synovitis. Note erosion of the lunate (hollow arrow) deep to the large crystal deposit. (B) Synovitis of the volar radiocarpal joint (asterisks) with dense ‘tophaceous’ appearing crystal deposits (white arrows). Note the hyperechoic rim over the lunate in the case of CPPD that resembles a double contour sign (hollow arrow) typically associated to gout. Example 2: flexor tenosynovitis and CPPD of the flexor tendon contributing to CTS. (C) Short axis view of the volar wrist at the level of the carpal tunnel shows tenosynovitis of the FPL and FCR tendons (white arrowheads) resulting in mass effect on the MN with convex appearance if the flexor retinaculum (open arrowheads). The hyperechoic appearance of the FPL tendon (white arrow) is secondary to CPPD. (D) Long axis view of the FPL tendon shows hypoechoic expansion of the tendon sheath from tenosynovitis (white arrowheads) contributing to median nerve compression. Example 3: CTS and compression and the medial nerve with CPPD and volar osteophytes and synovitis. (E) Long axis scan of the volar radiocarpal joint with and (F) without labels showing area of proliferative synovitis with punctate CPPD (dotted area) as well as a large radial osteophyte (white arrow) compressing the median nerve (white arrowheads) at the area entering the carpal tunnel. Example 4: synovitis and OA of the STT joint and CPPD in 2 different patients. (G) Long axis view of the volar wrist shows STT joint synovitis (asterisks) surrounding amorphous CPPD (white arrows) and adjacent erosions of the scaphoid (white arrowhead). Note distal tendinosis of the FCR tendon with hypoechoic thickening and loss of fibrillar pattern (open arrowheads). (H) Another example showing secondary osteoarthritis of the STT joint with large osteophytes of the scaphoid and trapezium (white arrowheads) and synovitis (asterisks) in a patient with confirmed CPPD disease and CTS. Example 5: cubital tunnel syndrome and CPPD. (I) Short axis view of the cubital tunnel of the medial elbow shows the mass effect on the UN resulting from synovitis and hyperemia (asterisks), globular CPP deposits (white arrows), and osteophytes of the humerus and olecranon (white arrowheads). (J) Corresponding long axis view of the UN being compressed (hollow arrows) as it traverses the cubital tunnel over the area of synovitis (asterisks), CPPD (white arrows), and osteophytes (white arrowheads). CPP: calcium pyrophosphate; CPPD: CPP deposition; CTS: carpal tunnel syndrome; FCR: flexor carpi radialis; FPL: flexor pollicis longus; FT: flexor tendon; MN: median nerve; OA: osteoarthritis; Rad: radius; STT: scaphotrapeziotrapezoid; UA: ulnar artery; UN: ulnar nerve
Ulnar nerve entrapment at the cubital tunnel has been reported in a small series of patients with CPPD with histopathologic evidence of CPP crystals and metaplasia of the chondrocytes. Calcification in the elbow has been noted to be present in the collateral ligaments, synovium, and hyaline cartilage, leading to ulnar nerve compression noted to occur between a fibrous band and a thickened ulnar collateral ligament, capsule, or region of osteophytes in the floor of the cubital tunnel (Figure 4I and J) [35].
Chronic CPPD disease is a prevalent condition closely associated with OA that is projected to gradually surge as the population over the age of 60 increases. The impact of CPPD leading to unusually extensive and severe OA has been broadly described in the literature. The underrecognized inflammatory elements leading to degenerative changes are best identified through advanced imaging and are commonly acknowledged at advanced stages of the disease after clinical consequences of structural damage develop. MRI and US are the best-suited imaging modalities to assess periarticular damage; with US having the advantage of simultaneously confirming the presence of pathogenic CPPD within the areas of inflammation and tissue damage. Routine US assessment of symptomatic joints in patients with CPPD disease can unveil a number of inflammatory findings as well as structural consequences that are logically expected to progress and lead to irreversible sequelae. Such is the case in SLAC wrist that frequently co-exists with advanced CPPD disease and leads as a common cause of wrist OA, chronic tenosynovitis leading to tendon rupture, and nerve damage from chronic CTS in CPPD disease. Current efforts to find effective calcium dissolving or targeted therapies to control inflammatory pathways in CPPD are underway, but to date, the effectiveness of proposed therapies has been inconsistent in achieving optimal disease control in chronic CPP arthritis. Recognizing the corollary inflammatory features of CPPD leading to tissue damage and their consequences should also create awareness of the need to reflect on preventive surgical interventions to mitigate the previously stated anticipated consequences of structural damage from CPPD disease until effective treatments emerge. Local osteophytes and conglomerated CPPD leading to chronic CTS are compressive elements that would not be amenable to medical therapy and may need consideration of surgical removal or decompression to avoid the consequences of chronic nerve damage. Furthermore, early preventive surgical intervention can be considered in cases with scapholunate dissociation and local identification of chronic inflammation with dense CPPD expected to progress to SLAC wrist and lead to severe wrist OA or necessitate more invasive surgical procedures. Lastly, it is important to consider the inclusion of structural damage characteristic of CPPD disease when developing scoring systems to stage disease severity and to acknowledge inflammatory features associated with CPPD for a global assessment of treatment response in chronic CPP arthritis.
CC: chondrocalcinosis
CPPD: calcium pyrophosphate deposition
CTS: carpal tunnel syndrome
MCP: metacarpophalangeal
OA: osteoarthritis
RA: rheumatoid arthritis
SLAC: scapholunate advance collapse
TFCC: fibrocartilage complex
US: ultrasound
JY: Conceptualization, Writing—original draft, Resources. MJ, PSK, and AG: Writing—review & editing.
Janeth Yinh is a consultant to Janssen, BICMD, and receives royalties from Springer Nature. Ali Guermazi is a consultant to Novartis, TissueGene, Coval, Medipost, 4Moving, Paradigm, Scarcell, Peptinov, Levicept, Pacira, ICM, and Formation Bio, he is a shareholder of BICL, LLC. Peter S. Kim is a consultant for Arthrex. Mohamed Jarraya has no relevant conflict of interest.
According to institutional patient compliance guidelines, since the cases presented are de-identified and not part of a research study, ethical approval is not required.
According to institutional patient compliance guidelines, since the cases presented are de-identified and not part of a research study, consent to participate is not required.
According to institutional patient compliance guidelines, since the cases presented are de-identified and not part of a research study, consent to publication is not required.
All datasets analyzed for this study are included in the manuscript.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2532
Download: 37
Times Cited: 0
Antje L Greenfield, Riti Kanesa-Thasan
Silvia Magni-Manzoni
Gregory J. Challener ... Minna J. Kohler
Jürgen Braun, Björn Bühring
