-
Exploration of Neuroscience
eISSN: 2834-5347EiC: Dirk M. Hermann, GermanyFrequency: Continuous PublicationAPC: No Article Processing Charge before September 30, 2027Publishing Model: Open AccessPeer Review Model: Single BlindPermanent Archive: PorticoIndexing & Archiving: Google Scholar, DOAJ, Dimensions, MyScienceWork, Portico, etc.Articles Celebrating the legacy of Russel J. Reiter: a pioneer in melatonin researchOpen AccessEditorialDirk M. Hermann, Ertugrul KilicPublished: March 24, 2025 Explor Neurosci. 2025;4:100679
Celebrating the legacy of Russel J. Reiter: a pioneer in melatonin researchOpen AccessEditorialDirk M. Hermann, Ertugrul KilicPublished: March 24, 2025 Explor Neurosci. 2025;4:100679
DOI: https://doi.org/10.37349/en.2025.100679
This article belongs to the special issue Identification of Therapeutic Targets in the Pathogenesis of Neurological Diseases Melatonin regulation of phase separation in Neuro-PASC: out-maneuvering Janus-faced amyloidsOpen AccessReviewThe SAR-CoV-2 virus has evolved to co-exist with human hosts, albeit at a substantial energetic cost resulting in post-infection neurological manifestations [Neuro-post-acute sequelae of SARS-CoV-2 [...] Read more.
Melatonin regulation of phase separation in Neuro-PASC: out-maneuvering Janus-faced amyloidsOpen AccessReviewThe SAR-CoV-2 virus has evolved to co-exist with human hosts, albeit at a substantial energetic cost resulting in post-infection neurological manifestations [Neuro-post-acute sequelae of SARS-CoV-2 [...] Read more.The SAR-CoV-2 virus has evolved to co-exist with human hosts, albeit at a substantial energetic cost resulting in post-infection neurological manifestations [Neuro-post-acute sequelae of SARS-CoV-2 infection (PASC)] that significantly impact public health and economic productivity on a global scale. One of the main molecular mechanisms responsible for the development of Neuro-PASC, in individuals of all ages, is the formation and inadequate proteolysis/clearance of phase-separated amyloid crystalline aggregates—a hallmark feature of aging-related neurodegenerative disorders. Amyloidogenesis during viral infection and persistence is a natural, inevitable, protective defense response that is exacerbated by SARS-CoV-2. Acting as chemical catalyst, SARS-CoV-2 accelerates hydrophobic collapse and the heterogeneous nucleation of amorphous amyloids into stable β-sheet aggregates. The clearance of amyloid aggregates is most effective during slow wave sleep, when high levels of adenosine triphosphate (ATP)—a biphasic modulator of biomolecular condensates—and melatonin are available to solubilize amyloid aggregates for removal. The dysregulation of mitochondrial dynamics by SARS-CoV-2, in particular fusion and fission homeostasis, impairs the proper formation of distinct mitochondrial subpopulations that can remedy challenges created by the diversion of substrates away from oxidative phosphorylation towards glycolysis to support viral replication and maintenance. The subsequent reduction of ATP and inhibition of melatonin synthesis during slow wave sleep results in incomplete brain clearance of amyloid aggregates, leading to the development of neurological manifestations commonly associated with age-related neurodegenerative disorders. Exogenous melatonin not only prevents mitochondrial dysfunction but also elevates ATP production, effectively augmenting the solubilizing effect of the adenosine moiety to ensure the timely, optimal disaggregation and clearance of pathogenic amyloid aggregates in the prevention and attenuation of Neuro-PASC.
Doris Loh, Russel J. ReiterPublished: March 24, 2025 Explor Neurosci. 2025;4:100678
DOI: https://doi.org/10.37349/en.2025.100678
This article belongs to the special issue Identification of Therapeutic Targets in the Pathogenesis of Neurological DiseasesThe SAR-CoV-2 virus has evolved to co-exist with human hosts, albeit at a substantial energetic cost resulting in post-infection neurological manifestations [Neuro-post-acute sequelae of SARS-CoV-2 infection (PASC)] that significantly impact public health and economic productivity on a global scale. One of the main molecular mechanisms responsible for the development of Neuro-PASC, in individuals of all ages, is the formation and inadequate proteolysis/clearance of phase-separated amyloid crystalline aggregates—a hallmark feature of aging-related neurodegenerative disorders. Amyloidogenesis during viral infection and persistence is a natural, inevitable, protective defense response that is exacerbated by SARS-CoV-2. Acting as chemical catalyst, SARS-CoV-2 accelerates hydrophobic collapse and the heterogeneous nucleation of amorphous amyloids into stable β-sheet aggregates. The clearance of amyloid aggregates is most effective during slow wave sleep, when high levels of adenosine triphosphate (ATP)—a biphasic modulator of biomolecular condensates—and melatonin are available to solubilize amyloid aggregates for removal. The dysregulation of mitochondrial dynamics by SARS-CoV-2, in particular fusion and fission homeostasis, impairs the proper formation of distinct mitochondrial subpopulations that can remedy challenges created by the diversion of substrates away from oxidative phosphorylation towards glycolysis to support viral replication and maintenance. The subsequent reduction of ATP and inhibition of melatonin synthesis during slow wave sleep results in incomplete brain clearance of amyloid aggregates, leading to the development of neurological manifestations commonly associated with age-related neurodegenerative disorders. Exogenous melatonin not only prevents mitochondrial dysfunction but also elevates ATP production, effectively augmenting the solubilizing effect of the adenosine moiety to ensure the timely, optimal disaggregation and clearance of pathogenic amyloid aggregates in the prevention and attenuation of Neuro-PASC.
 Raw resting-state electroencephalogram biomarker emerges as an indicator of Alzheimer’s disease over a two-year periodOpen AccessOriginal ArticleAim: Alzheimer’s disease (AD) is associated with several electrophysiological biomarkers. These biomarkers are associated with global decline in cognition and a diagnosis of AD. However, a spec [...] Read more.
Raw resting-state electroencephalogram biomarker emerges as an indicator of Alzheimer’s disease over a two-year periodOpen AccessOriginal ArticleAim: Alzheimer’s disease (AD) is associated with several electrophysiological biomarkers. These biomarkers are associated with global decline in cognition and a diagnosis of AD. However, a spec [...] Read more.Aim:
Alzheimer’s disease (AD) is associated with several electrophysiological biomarkers. These biomarkers are associated with global decline in cognition and a diagnosis of AD. However, a specific electrophysiological biomarker is not characterized as normal-functioning older adults convert to AD. The longitudinal retrospective study was conducted to describe an electrophysiological biomarker indicator for AD as normal-functioning older adults convert to a diagnosis in the AD continuum over a 2-year period.
Methods:
The study was conducted with 54 community-residing older adults, ranging from normal functioning to a diagnosis of AD. All initial and follow-up electrophysiological evaluations were completed in the New York University Brain Research Laboratories, and overall decline assessments with the Global Deterioration Scale (GDS) were completed in the New York University Aging and Dementia Research Center. Data included measurements from the GDS and raw resting-state electroencephalogram (rsEEG), which was transformed into quantitative EEG (qEEG) data. Data analysis consisted of descriptive statistics and a Kruskal-Wallis test. The level of significance was 0.05 with a moderate effect size. Topographic brain images displayed electrophysiological biomarkers.
Results:
A consistently increasing rsEEG theta frequency (P ≤ 0.01) occurred as normal-functioning older adults converted to AD across all GDS stages from the frontal to posterior regions with the progressive global decline. No discernible consistent electrophysiological changes were observed for rsEEG delta, alpha, or beta frequencies over all GDS stages. The GDS stages differed at baseline and follow-up (P ≤ 0.01). The rsEEG theta frequency increased with the progressive global decline across the GDS stages.
Conclusions:
The consistently increasing rsEEG theta frequency may be an electrophysiological biomarker indicator for AD from normal functioning to a diagnosis within the AD continuum. This biomarker will enhance the assessment of the risk, onset, and progression of AD and potentially inform the treatment of AD.
Ezra C. HolstonPublished: March 24, 2025 Explor Neurosci. 2025;4:100677
DOI: https://doi.org/10.37349/en.2025.100677
This article belongs to the special issue Alzheimer's DiseaseAim:
Alzheimer’s disease (AD) is associated with several electrophysiological biomarkers. These biomarkers are associated with global decline in cognition and a diagnosis of AD. However, a specific electrophysiological biomarker is not characterized as normal-functioning older adults convert to AD. The longitudinal retrospective study was conducted to describe an electrophysiological biomarker indicator for AD as normal-functioning older adults convert to a diagnosis in the AD continuum over a 2-year period.
Methods:
The study was conducted with 54 community-residing older adults, ranging from normal functioning to a diagnosis of AD. All initial and follow-up electrophysiological evaluations were completed in the New York University Brain Research Laboratories, and overall decline assessments with the Global Deterioration Scale (GDS) were completed in the New York University Aging and Dementia Research Center. Data included measurements from the GDS and raw resting-state electroencephalogram (rsEEG), which was transformed into quantitative EEG (qEEG) data. Data analysis consisted of descriptive statistics and a Kruskal-Wallis test. The level of significance was 0.05 with a moderate effect size. Topographic brain images displayed electrophysiological biomarkers.
Results:
A consistently increasing rsEEG theta frequency (P ≤ 0.01) occurred as normal-functioning older adults converted to AD across all GDS stages from the frontal to posterior regions with the progressive global decline. No discernible consistent electrophysiological changes were observed for rsEEG delta, alpha, or beta frequencies over all GDS stages. The GDS stages differed at baseline and follow-up (P ≤ 0.01). The rsEEG theta frequency increased with the progressive global decline across the GDS stages.
Conclusions:
The consistently increasing rsEEG theta frequency may be an electrophysiological biomarker indicator for AD from normal functioning to a diagnosis within the AD continuum. This biomarker will enhance the assessment of the risk, onset, and progression of AD and potentially inform the treatment of AD.
 Mechanisms of astrocytic and microglial purinergic signaling in homeostatic regulation and implications for neurological diseaseOpen AccessReviewPurinergic signaling, mediated by ATP and adenosine receptors, plays a crucial role in cellular communication and homeostasis within the central nervous system (CNS), particularly by regulating syna [...] Read more.
Mechanisms of astrocytic and microglial purinergic signaling in homeostatic regulation and implications for neurological diseaseOpen AccessReviewPurinergic signaling, mediated by ATP and adenosine receptors, plays a crucial role in cellular communication and homeostasis within the central nervous system (CNS), particularly by regulating syna [...] Read more.Purinergic signaling, mediated by ATP and adenosine receptors, plays a crucial role in cellular communication and homeostasis within the central nervous system (CNS), particularly by regulating synaptic activity, glial cell functions, and neuroplasticity. Glial cells, including astrocytes and microglia, contribute to both short-term processes, such as neurotransmission and neuroinflammation, and long-term functions, including synaptic remodeling, tissue repair, and behavioral adaptation. Dysregulation of purinergic signaling in these cells has been implicated in the pathogenesis of various neurodegenerative and neuropsychiatric disorders. This article explores the evolving concept of the synapse, highlighting the active role of glial cells in synaptic modulation and emphasizing the significance of purinergic signaling in synaptic function and responses to conditions such as injury and neurotoxicity. Specifically, it examines the roles of ATP and adenosine receptors—such as P2X4, P2X7, P2Y1, and P2Y12—in mediating key astrocytic and microglial functions, including neuroinflammation, phagocytosis, synaptic plasticity, and neuronal damage. Furthermore, the article discusses the involvement of purinergic receptors in neurological disorders such as epilepsy, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, ischemic stroke, Rett syndrome, and autism spectrum disorder, as well as potential therapeutic strategies targeting these receptors to mitigate inflammation, promote tissue repair, and improve clinical outcomes.
Moawiah M NaffaaPublished: March 11, 2025 Explor Neurosci. 2025;4:100676
DOI: https://doi.org/10.37349/en.2025.100676
This article belongs to the special issue Purinergic Signaling in Neuroinflammation and PainPurinergic signaling, mediated by ATP and adenosine receptors, plays a crucial role in cellular communication and homeostasis within the central nervous system (CNS), particularly by regulating synaptic activity, glial cell functions, and neuroplasticity. Glial cells, including astrocytes and microglia, contribute to both short-term processes, such as neurotransmission and neuroinflammation, and long-term functions, including synaptic remodeling, tissue repair, and behavioral adaptation. Dysregulation of purinergic signaling in these cells has been implicated in the pathogenesis of various neurodegenerative and neuropsychiatric disorders. This article explores the evolving concept of the synapse, highlighting the active role of glial cells in synaptic modulation and emphasizing the significance of purinergic signaling in synaptic function and responses to conditions such as injury and neurotoxicity. Specifically, it examines the roles of ATP and adenosine receptors—such as P2X4, P2X7, P2Y1, and P2Y12—in mediating key astrocytic and microglial functions, including neuroinflammation, phagocytosis, synaptic plasticity, and neuronal damage. Furthermore, the article discusses the involvement of purinergic receptors in neurological disorders such as epilepsy, Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, ischemic stroke, Rett syndrome, and autism spectrum disorder, as well as potential therapeutic strategies targeting these receptors to mitigate inflammation, promote tissue repair, and improve clinical outcomes.
 The role of neuroimaging in Alzheimer’s disease: implications for the diagnosis, monitoring disease progression, and treatmentOpen AccessReviewAlzheimer’s disease (AD) is a neurodegenerative disorder that affects millions of people worldwide. It presents a significant challenge in terms of accurate diagnosis, disease progression monitori [...] Read more.
The role of neuroimaging in Alzheimer’s disease: implications for the diagnosis, monitoring disease progression, and treatmentOpen AccessReviewAlzheimer’s disease (AD) is a neurodegenerative disorder that affects millions of people worldwide. It presents a significant challenge in terms of accurate diagnosis, disease progression monitori [...] Read more.Alzheimer’s disease (AD) is a neurodegenerative disorder that affects millions of people worldwide. It presents a significant challenge in terms of accurate diagnosis, disease progression monitoring, and the development of effective treatments. This article addresses the role of neuroimaging as an advancing tool for diagnosis, monitoring progression, and treatment of AD. A comprehensive review of existing literature on the use of neuroimaging in AD was conducted using various databases. The different imaging techniques, such as magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET), were examined in terms of their ability to detect amyloid beta (Aβ) plaques and neurofibrillary tangles (NFTs), the hallmark pathological features of AD. Neuroimaging enables the visualization of Alzheimer-related biomarkers, such as Aβ plaques, tau protein tangles
, neuro-inflammation, and synaptic dysfunction, providing valuable insights into disease pathophysiology and progression. These imaging techniques assist in the early detection of AD, distinguishing it from other conditions and evaluating the effectiveness of treatments. This has the potential to significantly transform the way AD is managed clinically. By providing insights into the molecular changes that occur in the brain during the course of the disease, neuroimaging can facilitate early diagnosis, monitor disease progression, and inform treatment decisions. Furthermore, neuroimaging holds great potential for accelerating drug development by allowing researchers to assess the efficacy of novel therapies in real time. Overall, the integration of neuroimaging into the clinical management of AD has the potential to revolutionize the way we approach diagnosis, treatment, and research in AD.Julius Mulumba ... Yong YangPublished: February 25, 2025 Explor Neurosci. 2025;4:100675
DOI: https://doi.org/10.37349/en.2025.100675
This article belongs to the special issue Alzheimer's DiseaseAlzheimer’s disease (AD) is a neurodegenerative disorder that affects millions of people worldwide. It presents a significant challenge in terms of accurate diagnosis, disease progression monitoring, and the development of effective treatments. This article addresses the role of neuroimaging as an advancing tool for diagnosis, monitoring progression, and treatment of AD. A comprehensive review of existing literature on the use of neuroimaging in AD was conducted using various databases. The different imaging techniques, such as magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET), were examined in terms of their ability to detect amyloid beta (Aβ) plaques and neurofibrillary tangles (NFTs), the hallmark pathological features of AD. Neuroimaging enables the visualization of Alzheimer-related biomarkers, such as Aβ plaques, tau protein tangles
, neuro-inflammation, and synaptic dysfunction, providing valuable insights into disease pathophysiology and progression. These imaging techniques assist in the early detection of AD, distinguishing it from other conditions and evaluating the effectiveness of treatments. This has the potential to significantly transform the way AD is managed clinically. By providing insights into the molecular changes that occur in the brain during the course of the disease, neuroimaging can facilitate early diagnosis, monitor disease progression, and inform treatment decisions. Furthermore, neuroimaging holds great potential for accelerating drug development by allowing researchers to assess the efficacy of novel therapies in real time. Overall, the integration of neuroimaging into the clinical management of AD has the potential to revolutionize the way we approach diagnosis, treatment, and research in AD. Neurostimulation devices to treat Alzheimer’s diseaseOpen AccessReviewThe use of neurostimulation devices for the treatment of Alzheimer’s disease (AD) is a growing field. In this review, we examine the mechanism of action and therapeutic indications of these neuros [...] Read more.
Neurostimulation devices to treat Alzheimer’s diseaseOpen AccessReviewThe use of neurostimulation devices for the treatment of Alzheimer’s disease (AD) is a growing field. In this review, we examine the mechanism of action and therapeutic indications of these neuros [...] Read more.The use of neurostimulation devices for the treatment of Alzheimer’s disease (AD) is a growing field. In this review, we examine the mechanism of action and therapeutic indications of these neurostimulation devices in the AD process. Rapid advancements in neurostimulation technologies are providing non-pharmacological relief to patients affected by AD pathology. Neurostimulation therapies include electrical stimulation that targets the circuitry-level connection in important brain areas such as the hippocampus to induce therapeutic neuromodulation of dysfunctional neural circuitry and electromagnetic field (EMF) stimulation that targets anti-amyloid molecular pathways to promote the degradation of beta-amyloid (Aβ). These devices target specific or diffuse cortical and subcortical brain areas to modulate neuronal activity at the electrophysiological or molecular pathway level, providing therapeutic effects for AD. This review attempts to determine the most effective and safe neurostimulation device for AD and provides an overview of potential and current clinical indications. Several EMF devices have shown a beneficial or harmful effect in cell cultures and animal models but not in AD human studies. These contradictory results may be related to the stimulation parameters of these devices, such as frequency, penetration depth, power deposition measured by specific absorption rate, time of exposure, type of cell, and tissue dielectric properties. Based on this, determining the optimal stimulation parameters for EMF devices in AD and understanding their mechanism of action is essential to promote their clinical application, our review suggests that repeated EMF stimulation (REMFS) is the most appropriate device for human AD treatments. Before its clinical application, it is necessary to consider the complicated and interconnected genetic and epigenetic effects of REMFS-biological system interaction. This will move forward the urgently needed therapy of EMF in human AD.
Felipe P. Perez ... Maher RizkallaPublished: February 25, 2025 Explor Neurosci. 2025;4:100674
DOI: https://doi.org/10.37349/en.2025.100674
This article belongs to the special issue Alzheimer's DiseaseThe use of neurostimulation devices for the treatment of Alzheimer’s disease (AD) is a growing field. In this review, we examine the mechanism of action and therapeutic indications of these neurostimulation devices in the AD process. Rapid advancements in neurostimulation technologies are providing non-pharmacological relief to patients affected by AD pathology. Neurostimulation therapies include electrical stimulation that targets the circuitry-level connection in important brain areas such as the hippocampus to induce therapeutic neuromodulation of dysfunctional neural circuitry and electromagnetic field (EMF) stimulation that targets anti-amyloid molecular pathways to promote the degradation of beta-amyloid (Aβ). These devices target specific or diffuse cortical and subcortical brain areas to modulate neuronal activity at the electrophysiological or molecular pathway level, providing therapeutic effects for AD. This review attempts to determine the most effective and safe neurostimulation device for AD and provides an overview of potential and current clinical indications. Several EMF devices have shown a beneficial or harmful effect in cell cultures and animal models but not in AD human studies. These contradictory results may be related to the stimulation parameters of these devices, such as frequency, penetration depth, power deposition measured by specific absorption rate, time of exposure, type of cell, and tissue dielectric properties. Based on this, determining the optimal stimulation parameters for EMF devices in AD and understanding their mechanism of action is essential to promote their clinical application, our review suggests that repeated EMF stimulation (REMFS) is the most appropriate device for human AD treatments. Before its clinical application, it is necessary to consider the complicated and interconnected genetic and epigenetic effects of REMFS-biological system interaction. This will move forward the urgently needed therapy of EMF in human AD.
 Current therapeutics for Alzheimer’s disease and clinical trialsOpen AccessReviewAlzheimer’s disease (AD) is a major type of dementia and neurodegenerative disease, characterized by memory loss and cognitive decline. Over decades, significant efforts have been dedicated to fin [...] Read more.
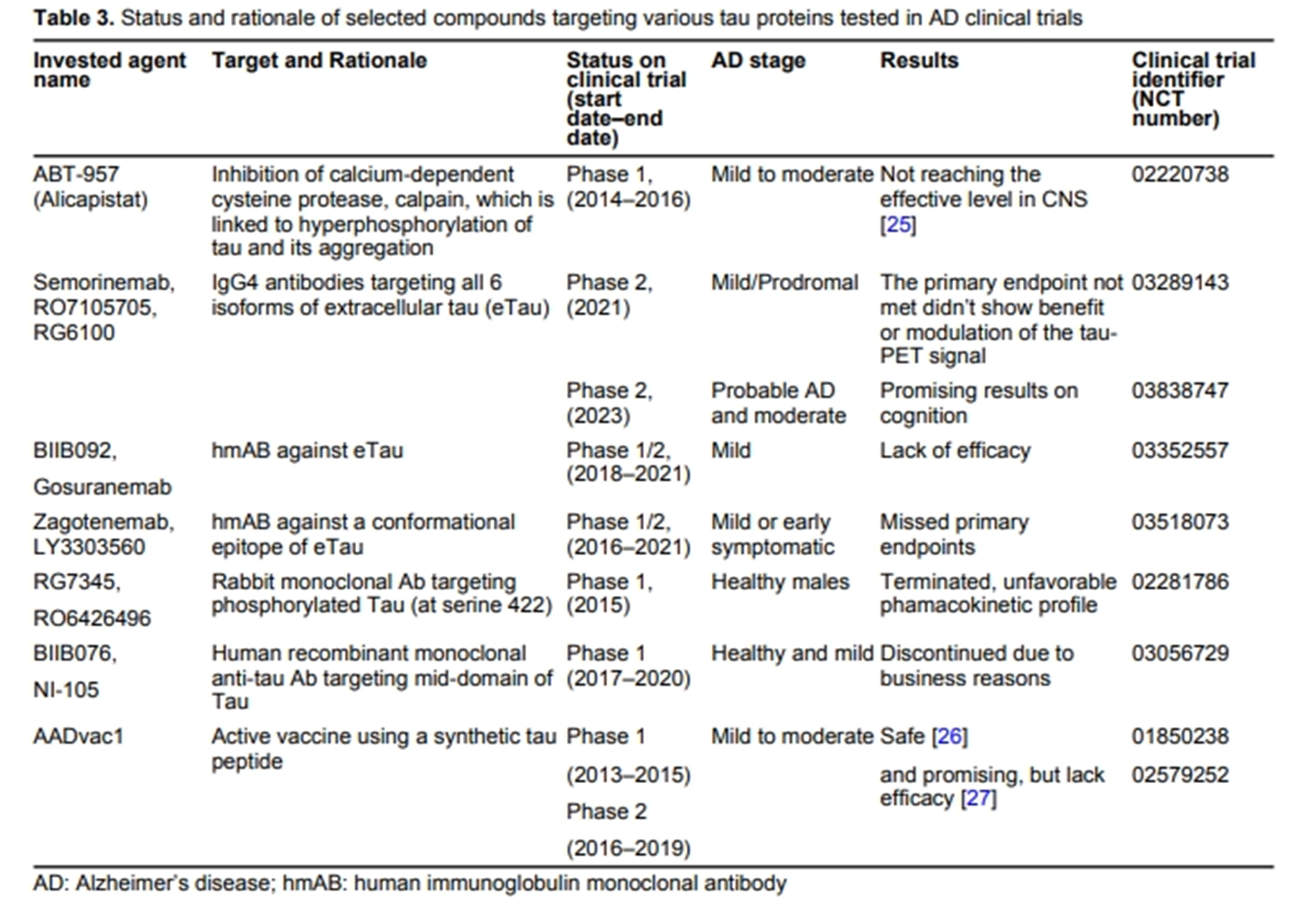
Current therapeutics for Alzheimer’s disease and clinical trialsOpen AccessReviewAlzheimer’s disease (AD) is a major type of dementia and neurodegenerative disease, characterized by memory loss and cognitive decline. Over decades, significant efforts have been dedicated to fin [...] Read more.Alzheimer’s disease (AD) is a major type of dementia and neurodegenerative disease, characterized by memory loss and cognitive decline. Over decades, significant efforts have been dedicated to finding its cause, pathogenic mechanisms, biomarkers for early detection, and clinical trials for its treatment. Earlier approved drugs mainly ameliorated the symptoms of AD, until recent years when two drugs targeting amyloid-beta (Aβ) protein were approved to slow down the progression of the disease. This review article encompasses the history of drug development in treating AD and clinical trials that failed and succeeded. Clinicaltrials.org website was systematically searched and screened for randomized controlled trials with results posted in the past 10 years. Among the 3,388 AD clinical trials, 211 interventional studies registered under AD have met eligibility. This review includes the interventional targets for drug discovery such as Aβ, tau, neurotransmitter receptors, neuroinflammation, multi-target studies, repurposing pharmacological agents, non-pharmacological interventions, and clinical therapy development for the neuropsychiatric symptoms of dementia. Current clinical trials are ongoing and no results are available as of yet. With the vast choices of drug targets that have been investigated, this review aims to present some insights into future AD drug design and trials and contribute to our ongoing efforts to find the cure.
Danqing Xiao, Chen ZhangPublished: June 27, 2024 Explor Neurosci. 2024;3:255–271
DOI: https://doi.org/10.37349/en.2024.00048
This article belongs to the special issue Alzheimer’s DiseaseAlzheimer’s disease (AD) is a major type of dementia and neurodegenerative disease, characterized by memory loss and cognitive decline. Over decades, significant efforts have been dedicated to finding its cause, pathogenic mechanisms, biomarkers for early detection, and clinical trials for its treatment. Earlier approved drugs mainly ameliorated the symptoms of AD, until recent years when two drugs targeting amyloid-beta (Aβ) protein were approved to slow down the progression of the disease. This review article encompasses the history of drug development in treating AD and clinical trials that failed and succeeded. Clinicaltrials.org website was systematically searched and screened for randomized controlled trials with results posted in the past 10 years. Among the 3,388 AD clinical trials, 211 interventional studies registered under AD have met eligibility. This review includes the interventional targets for drug discovery such as Aβ, tau, neurotransmitter receptors, neuroinflammation, multi-target studies, repurposing pharmacological agents, non-pharmacological interventions, and clinical therapy development for the neuropsychiatric symptoms of dementia. Current clinical trials are ongoing and no results are available as of yet. With the vast choices of drug targets that have been investigated, this review aims to present some insights into future AD drug design and trials and contribute to our ongoing efforts to find the cure.
 Negative environmental influences on the developing brain mediated by epigenetic modificationsOpen AccessReviewBrain development, a complex process, consisting of several phases, starting as early as two weeks after conception, and continuing through childhood till early adolescence, is crucial for the devel [...] Read more.
Negative environmental influences on the developing brain mediated by epigenetic modificationsOpen AccessReviewBrain development, a complex process, consisting of several phases, starting as early as two weeks after conception, and continuing through childhood till early adolescence, is crucial for the devel [...] Read more.Brain development, a complex process, consisting of several phases, starting as early as two weeks after conception, and continuing through childhood till early adolescence, is crucial for the development of properly functioning body systems, behavioral traits, and neurocognitive abilities. Infancy and childhood are recognized as important periods for initial brain formation, however in later stages of life, such as childhood and adulthood, experiences, together with environmental exposures, can still influence brain physiology. The developing brain is particularly susceptible to epigenetic changes with many factors being proposed as modifiers by directly impacting DNA methylation as well as histone and chromatin modifications within genes implicated in development. These factors include: maternal stress and diet, exposure to pollutants, sleep quality, as well as dietary habits. Evidence indicates exposures to environmental threats can lead to inappropriate neurological, metabolic, and endocrine functioning often mediated by epigenetic mechanisms with symptoms manifesting themselves as early as childhood or in later stages of life. Therefore, the main aim of this review is to evaluate the current studies focused on negative environmental exposures and their consequences on the developing brain directed by epigenetic mechanisms.
Maya Komar-Fletcher ... Joanna Michalina JurekPublished: September 28, 2023 Explor Neurosci. 2023;2:193–211
DOI: https://doi.org/10.37349/en.2023.00021Brain development, a complex process, consisting of several phases, starting as early as two weeks after conception, and continuing through childhood till early adolescence, is crucial for the development of properly functioning body systems, behavioral traits, and neurocognitive abilities. Infancy and childhood are recognized as important periods for initial brain formation, however in later stages of life, such as childhood and adulthood, experiences, together with environmental exposures, can still influence brain physiology. The developing brain is particularly susceptible to epigenetic changes with many factors being proposed as modifiers by directly impacting DNA methylation as well as histone and chromatin modifications within genes implicated in development. These factors include: maternal stress and diet, exposure to pollutants, sleep quality, as well as dietary habits. Evidence indicates exposures to environmental threats can lead to inappropriate neurological, metabolic, and endocrine functioning often mediated by epigenetic mechanisms with symptoms manifesting themselves as early as childhood or in later stages of life. Therefore, the main aim of this review is to evaluate the current studies focused on negative environmental exposures and their consequences on the developing brain directed by epigenetic mechanisms.
 Impact of circadian clock dysfunction on human healthOpen AccessReviewAll living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic act [...] Read more.
Impact of circadian clock dysfunction on human healthOpen AccessReviewAll living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic act [...] Read more.All living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic activity, heart rate variability, hormone secretion, and others. The hypothalamic suprachiasmatic nucleus (SCN) acts as a primary circadian pacemaker. Peripheral tissues have an endogenous circadian clock; however, SCN synchronizes the circadian activity of the peripheral clocks. The retinohypothalamic tract (RHT) from retinal ganglionic cells carries the photic signal into the SCN that regulates the rhythmic expression of the core clock genes through the feedback loop. At the output level, the SCN connects with the pineal gland and the peripheral tissues with the help of neuroendocrine mediators. Disruption of circadian clock functions is detrimental to health. Shift work, night work, chronic or acute jet lag, and light-at-night have adverse effects on circadian functions. Misalignment of circadian rhythm alters the expression of core clock genes, leading to deregulation of cellular activity and metabolic functions. Circadian rhythm dysfunction causes many pathologic conditions, including sleep disorders, cardiovascular problems, metabolic dysfunction, infertility, poor physical performance, as well as cancer. The present work has reviewed the relationship between circadian clock dysfunction and impaired physiological activities.
Saptadip Samanta, Sk Asif AliPublished: September 29, 2022 Explor Neurosci. 2022;1:4–30
DOI: https://doi.org/10.37349/en.2022.00002
This article belongs to the special issue Circadian Rhythm and MelatoninAll living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic activity, heart rate variability, hormone secretion, and others. The hypothalamic suprachiasmatic nucleus (SCN) acts as a primary circadian pacemaker. Peripheral tissues have an endogenous circadian clock; however, SCN synchronizes the circadian activity of the peripheral clocks. The retinohypothalamic tract (RHT) from retinal ganglionic cells carries the photic signal into the SCN that regulates the rhythmic expression of the core clock genes through the feedback loop. At the output level, the SCN connects with the pineal gland and the peripheral tissues with the help of neuroendocrine mediators. Disruption of circadian clock functions is detrimental to health. Shift work, night work, chronic or acute jet lag, and light-at-night have adverse effects on circadian functions. Misalignment of circadian rhythm alters the expression of core clock genes, leading to deregulation of cellular activity and metabolic functions. Circadian rhythm dysfunction causes many pathologic conditions, including sleep disorders, cardiovascular problems, metabolic dysfunction, infertility, poor physical performance, as well as cancer. The present work has reviewed the relationship between circadian clock dysfunction and impaired physiological activities.
 Stigma and psychosocial problems in patients with epilepsyOpen AccessReviewEpilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients [...] Read more.
Stigma and psychosocial problems in patients with epilepsyOpen AccessReviewEpilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients [...] Read more.Epilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients to enjoy a seizure-free life. However, throughout history, epilepsy has acquired diverse interpretations due to the experienced seizures, transforming the condition from a clinical issue into a social stigma. Therefore, the aim of this review study is to review stigma and psychosocial problems in patients with epilepsy (PwE). For this reason, this study utilises sources from the last ten years and reports current data. As a result of the review, it was found that societal discrimination in PwE arises primarily from inadequate knowledge, misconceptions, and negative attitudes toward the condition. Other contributing factors were include patients’ lower levels of education and income, frequent seizures due to inadequate treatment, age at onset, duration of the disease, depressive symptoms, and lack of social support. Also, it was found that the stigma individuals with epilepsy face plays a pivotal role in exacerbating their psychosocial problems. Unfortunately, stigma and psychosocial challenges appear to be in a vicious circle, with an increase in one increasing the other. Stigmatized patients tended to isolate themselves from society, further increasing their likelihood of experiencing a depressive mood or psychiatric comorbidity. Consequently, individuals with epilepsy encounter difficulties in various domains such as marriage, work, education, and personal life. Considering these significant psychosocial burdens, it is essential to recognize that epilepsy surpasses its medical implications. Unfortunately, current efforts to reduce stigma remain insufficient, necessitating urgent and comprehensive measures to address this issue.
Kubra YeniPublished: December 06, 2023 Explor Neurosci. 2023;2:251–263
DOI: https://doi.org/10.37349/en.2023.00026
This article belongs to the special issue EpilepsyEpilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients to enjoy a seizure-free life. However, throughout history, epilepsy has acquired diverse interpretations due to the experienced seizures, transforming the condition from a clinical issue into a social stigma. Therefore, the aim of this review study is to review stigma and psychosocial problems in patients with epilepsy (PwE). For this reason, this study utilises sources from the last ten years and reports current data. As a result of the review, it was found that societal discrimination in PwE arises primarily from inadequate knowledge, misconceptions, and negative attitudes toward the condition. Other contributing factors were include patients’ lower levels of education and income, frequent seizures due to inadequate treatment, age at onset, duration of the disease, depressive symptoms, and lack of social support. Also, it was found that the stigma individuals with epilepsy face plays a pivotal role in exacerbating their psychosocial problems. Unfortunately, stigma and psychosocial challenges appear to be in a vicious circle, with an increase in one increasing the other. Stigmatized patients tended to isolate themselves from society, further increasing their likelihood of experiencing a depressive mood or psychiatric comorbidity. Consequently, individuals with epilepsy encounter difficulties in various domains such as marriage, work, education, and personal life. Considering these significant psychosocial burdens, it is essential to recognize that epilepsy surpasses its medical implications. Unfortunately, current efforts to reduce stigma remain insufficient, necessitating urgent and comprehensive measures to address this issue.
 Update for astrocytomas: medical and surgical management considerationsOpen AccessReviewAstrocytomas include a wide range of tumors with unique mutations and varying grades of malignancy. These tumors all originate from the astrocyte, a star-shaped glial cell that plays a major role in [...] Read more.
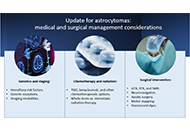
Update for astrocytomas: medical and surgical management considerationsOpen AccessReviewAstrocytomas include a wide range of tumors with unique mutations and varying grades of malignancy. These tumors all originate from the astrocyte, a star-shaped glial cell that plays a major role in [...] Read more.Astrocytomas include a wide range of tumors with unique mutations and varying grades of malignancy. These tumors all originate from the astrocyte, a star-shaped glial cell that plays a major role in supporting functions of the central nervous system (CNS), including blood-brain barrier (BBB) development and maintenance, water and ion regulation, influencing neuronal synaptogenesis, and stimulating the immunological response. In terms of epidemiology, glioblastoma (GB), the most common and malignant astrocytoma, generally occur with higher rates in Australia, Western Europe, and Canada, with the lowest rates in Southeast Asia. Additionally, significantly higher rates of GB are observed in males and non-Hispanic whites. It has been suggested that higher levels of testosterone observed in biological males may account for the increased rates of GB. Hereditary syndromes such as Cowden, Lynch, Turcot, Li-Fraumeni, and neurofibromatosis type 1 have been linked to increased rates of astrocytoma development. While there are a number of specific gene mutations that may influence malignancy or be targeted in astrocytoma treatment, O6-methylguanine-DNA methyltransferase (MGMT) gene function is an important predictor of astrocytoma response to chemotherapeutic agent temozolomide (TMZ). TMZ for primary and bevacizumab in the setting of recurrent tumor formation are two of the main chemotherapeutic agents currently approved in the treatment of astrocytomas. While stereotactic radiosurgery (SRS) has debatable implications for increased survival in comparison to whole-brain radiotherapy (WBRT), SRS demonstrates increased precision with reduced radiation toxicity. When considering surgical resection of astrocytoma, the extent of resection (EoR) is taken into consideration. Subtotal resection (STR) spares the margins of the T1 enhanced magnetic resonance imaging (MRI) region, gross total resection (GTR) includes the margins, and supramaximal resection (SMR) extends beyond the margin of the T1 and into the T2 region. Surgical resection, radiation, and chemotherapy are integral components of astrocytoma treatment.
Hereditary risk factors, genetic mutations, and imaging modalities are discussed in reference to astrocytoma staging and mechanism of growth. In terms of the treatment of astrocytomas, chemotherapy, radiation therapy, and strategic surgical interventions are discussed
Matthew Willman ... Brandon Lucke-WoldPublished: February 23, 2023 Explor Neurosci. 2023;2:1–26
DOI: https://doi.org/10.37349/en.2023.00009Astrocytomas include a wide range of tumors with unique mutations and varying grades of malignancy. These tumors all originate from the astrocyte, a star-shaped glial cell that plays a major role in supporting functions of the central nervous system (CNS), including blood-brain barrier (BBB) development and maintenance, water and ion regulation, influencing neuronal synaptogenesis, and stimulating the immunological response. In terms of epidemiology, glioblastoma (GB), the most common and malignant astrocytoma, generally occur with higher rates in Australia, Western Europe, and Canada, with the lowest rates in Southeast Asia. Additionally, significantly higher rates of GB are observed in males and non-Hispanic whites. It has been suggested that higher levels of testosterone observed in biological males may account for the increased rates of GB. Hereditary syndromes such as Cowden, Lynch, Turcot, Li-Fraumeni, and neurofibromatosis type 1 have been linked to increased rates of astrocytoma development. While there are a number of specific gene mutations that may influence malignancy or be targeted in astrocytoma treatment, O6-methylguanine-DNA methyltransferase (MGMT) gene function is an important predictor of astrocytoma response to chemotherapeutic agent temozolomide (TMZ). TMZ for primary and bevacizumab in the setting of recurrent tumor formation are two of the main chemotherapeutic agents currently approved in the treatment of astrocytomas. While stereotactic radiosurgery (SRS) has debatable implications for increased survival in comparison to whole-brain radiotherapy (WBRT), SRS demonstrates increased precision with reduced radiation toxicity. When considering surgical resection of astrocytoma, the extent of resection (EoR) is taken into consideration. Subtotal resection (STR) spares the margins of the T1 enhanced magnetic resonance imaging (MRI) region, gross total resection (GTR) includes the margins, and supramaximal resection (SMR) extends beyond the margin of the T1 and into the T2 region. Surgical resection, radiation, and chemotherapy are integral components of astrocytoma treatment.
Hereditary risk factors, genetic mutations, and imaging modalities are discussed in reference to astrocytoma staging and mechanism of growth. In terms of the treatment of astrocytomas, chemotherapy, radiation therapy, and strategic surgical interventions are discussed
 Neuroprotective compounds from three common medicinal plants of West Bengal, India: a mini reviewOpen AccessMini ReviewNeural disorders refer to conditions of the nervous system due to infection or degeneration of the neurons leading to either neurodegenerative disorder or neuropsychiatric disorder. Some such disord [...] Read more.
Neuroprotective compounds from three common medicinal plants of West Bengal, India: a mini reviewOpen AccessMini ReviewNeural disorders refer to conditions of the nervous system due to infection or degeneration of the neurons leading to either neurodegenerative disorder or neuropsychiatric disorder. Some such disord [...] Read more.Neural disorders refer to conditions of the nervous system due to infection or degeneration of the neurons leading to either neurodegenerative disorder or neuropsychiatric disorder. Some such disorders of the nervous system include Parkinsons’s disease, depression, amnesia, dementia, Alzheimer’s disease, schizophrenia, cerebrovascular impairment, epilepsy, seizure disorders, etc. In conventional medical system, some medicines belonging to the class of psychodelic drugs, sedatives, neurotransmitters, neuro-stimulants, etc. are in extensive use. Unfortunately, most of these drugs either delay the progression of the neural disorder or leave the patient with prominent adverse side effects. Several potent bioactive compounds with neuroprotective potential have been reported from medicinal plants and some of them have been found to be highly effective. Belonging from natural sources, mostly, the plant derived compounds exhibit minimum or no cytotoxicity at a prescribed standardised dose against a particular health ailment. Many such phytocompounds from plant sources with potent neuroprotective activities have been in use in Ayurvedacharya, Unani, and Chinese medicine for ages. The compounds if isolated chemically, modified to make more potent neuroprotective derivatives and utilised to make highly effective neuroprotective pharmaceutical formulations with minimum side effects, may open new revolutionary doorways in neuropharmacology. In this review, it has been briefly discussed about the neuroprotective compounds isolated from certain indigenous plants of West Bengal, India, and their mechanism of action.
Suvendu Ghosh ... Debosree GhoshPublished: December 26, 2023 Explor Neurosci. 2023;2:307–317
DOI: https://doi.org/10.37349/en.2023.00030
This article belongs to the special issue Medicinal Plants and Bioactive Phytochemicals in NeuroprotectionNeural disorders refer to conditions of the nervous system due to infection or degeneration of the neurons leading to either neurodegenerative disorder or neuropsychiatric disorder. Some such disorders of the nervous system include Parkinsons’s disease, depression, amnesia, dementia, Alzheimer’s disease, schizophrenia, cerebrovascular impairment, epilepsy, seizure disorders, etc. In conventional medical system, some medicines belonging to the class of psychodelic drugs, sedatives, neurotransmitters, neuro-stimulants, etc. are in extensive use. Unfortunately, most of these drugs either delay the progression of the neural disorder or leave the patient with prominent adverse side effects. Several potent bioactive compounds with neuroprotective potential have been reported from medicinal plants and some of them have been found to be highly effective. Belonging from natural sources, mostly, the plant derived compounds exhibit minimum or no cytotoxicity at a prescribed standardised dose against a particular health ailment. Many such phytocompounds from plant sources with potent neuroprotective activities have been in use in Ayurvedacharya, Unani, and Chinese medicine for ages. The compounds if isolated chemically, modified to make more potent neuroprotective derivatives and utilised to make highly effective neuroprotective pharmaceutical formulations with minimum side effects, may open new revolutionary doorways in neuropharmacology. In this review, it has been briefly discussed about the neuroprotective compounds isolated from certain indigenous plants of West Bengal, India, and their mechanism of action.
 Nutritional treatment with the ketogenic diet in children with refractory epilepsy: a narrative reviewOpen AccessMini ReviewThe two mainstays of therapy for refractory epilepsy are medication and surgery. Child behavioral and cognitive aspects of epilepsy can be improved by using a specialized dietary regimen such as the [...] Read more.
Nutritional treatment with the ketogenic diet in children with refractory epilepsy: a narrative reviewOpen AccessMini ReviewThe two mainstays of therapy for refractory epilepsy are medication and surgery. Child behavioral and cognitive aspects of epilepsy can be improved by using a specialized dietary regimen such as the [...] Read more.The two mainstays of therapy for refractory epilepsy are medication and surgery. Child behavioral and cognitive aspects of epilepsy can be improved by using a specialized dietary regimen such as the ketogenic diet (KD). The purpose of this review is to expand our understanding of KD as a nutritional therapy for children with refractory epilepsy and to provide insight into the physiological aspects of its efficacy as an alternative to anti-seizure medication. Either directly or indirectly, ketones, glucose restriction, and polyunsaturated fatty acids regulate epileptic seizures. For KD to be effective, all three of these components must be present, even though the exact mechanism is unknown. Increasing gamma-aminobutyric acid, mitochondrial biogenesis, and oxidative phosphorylation levels can also serve as a means of promoting stable synaptic function while also decreasing neural activity and excitability. Most side effects of KD are caused by mild metabolic abnormalities such as acidosis, hyperuricemia, hypercholesterolemia, hypocalcemia, and hypomagnesemia. Since medium-chain triglycerides (MCTs) produce more ketones per calorie than long-chain triglycerides, individuals who consume MCTs can consume more carbohydrates and protein. This review demonstrated that KD therapy led to positive outcomes for patients with refractory epilepsy. Further study is needed to evaluate whether less restrictive and easier-to-follow diets, such as the modified Atkins diet and MCT diets, have a similar effect on seizure treatment as the standard KD.
Srilaxmi Vityala ... Swathi NenavathPublished: October 30, 2023 Explor Neurosci. 2023;2:245–250
DOI: https://doi.org/10.37349/en.2023.00025
This article belongs to the special issue EpilepsyThe two mainstays of therapy for refractory epilepsy are medication and surgery. Child behavioral and cognitive aspects of epilepsy can be improved by using a specialized dietary regimen such as the ketogenic diet (KD). The purpose of this review is to expand our understanding of KD as a nutritional therapy for children with refractory epilepsy and to provide insight into the physiological aspects of its efficacy as an alternative to anti-seizure medication. Either directly or indirectly, ketones, glucose restriction, and polyunsaturated fatty acids regulate epileptic seizures. For KD to be effective, all three of these components must be present, even though the exact mechanism is unknown. Increasing gamma-aminobutyric acid, mitochondrial biogenesis, and oxidative phosphorylation levels can also serve as a means of promoting stable synaptic function while also decreasing neural activity and excitability. Most side effects of KD are caused by mild metabolic abnormalities such as acidosis, hyperuricemia, hypercholesterolemia, hypocalcemia, and hypomagnesemia. Since medium-chain triglycerides (MCTs) produce more ketones per calorie than long-chain triglycerides, individuals who consume MCTs can consume more carbohydrates and protein. This review demonstrated that KD therapy led to positive outcomes for patients with refractory epilepsy. Further study is needed to evaluate whether less restrictive and easier-to-follow diets, such as the modified Atkins diet and MCT diets, have a similar effect on seizure treatment as the standard KD.
 Current therapeutics for Alzheimer’s disease and clinical trialsOpen AccessReviewAlzheimer’s disease (AD) is a major type of dementia and neurodegenerative disease, characterized by memory loss and cognitive decline. Over decades, significant efforts have been dedicated to fin [...] Read more.
Current therapeutics for Alzheimer’s disease and clinical trialsOpen AccessReviewAlzheimer’s disease (AD) is a major type of dementia and neurodegenerative disease, characterized by memory loss and cognitive decline. Over decades, significant efforts have been dedicated to fin [...] Read more.Alzheimer’s disease (AD) is a major type of dementia and neurodegenerative disease, characterized by memory loss and cognitive decline. Over decades, significant efforts have been dedicated to finding its cause, pathogenic mechanisms, biomarkers for early detection, and clinical trials for its treatment. Earlier approved drugs mainly ameliorated the symptoms of AD, until recent years when two drugs targeting amyloid-beta (Aβ) protein were approved to slow down the progression of the disease. This review article encompasses the history of drug development in treating AD and clinical trials that failed and succeeded. Clinicaltrials.org website was systematically searched and screened for randomized controlled trials with results posted in the past 10 years. Among the 3,388 AD clinical trials, 211 interventional studies registered under AD have met eligibility. This review includes the interventional targets for drug discovery such as Aβ, tau, neurotransmitter receptors, neuroinflammation, multi-target studies, repurposing pharmacological agents, non-pharmacological interventions, and clinical therapy development for the neuropsychiatric symptoms of dementia. Current clinical trials are ongoing and no results are available as of yet. With the vast choices of drug targets that have been investigated, this review aims to present some insights into future AD drug design and trials and contribute to our ongoing efforts to find the cure.
Danqing Xiao, Chen ZhangPublished: June 27, 2024 Explor Neurosci. 2024;3:255–271
DOI: https://doi.org/10.37349/en.2024.00048
This article belongs to the special issue Alzheimer’s DiseaseAlzheimer’s disease (AD) is a major type of dementia and neurodegenerative disease, characterized by memory loss and cognitive decline. Over decades, significant efforts have been dedicated to finding its cause, pathogenic mechanisms, biomarkers for early detection, and clinical trials for its treatment. Earlier approved drugs mainly ameliorated the symptoms of AD, until recent years when two drugs targeting amyloid-beta (Aβ) protein were approved to slow down the progression of the disease. This review article encompasses the history of drug development in treating AD and clinical trials that failed and succeeded. Clinicaltrials.org website was systematically searched and screened for randomized controlled trials with results posted in the past 10 years. Among the 3,388 AD clinical trials, 211 interventional studies registered under AD have met eligibility. This review includes the interventional targets for drug discovery such as Aβ, tau, neurotransmitter receptors, neuroinflammation, multi-target studies, repurposing pharmacological agents, non-pharmacological interventions, and clinical therapy development for the neuropsychiatric symptoms of dementia. Current clinical trials are ongoing and no results are available as of yet. With the vast choices of drug targets that have been investigated, this review aims to present some insights into future AD drug design and trials and contribute to our ongoing efforts to find the cure.
 Stigma and psychosocial problems in patients with epilepsyOpen AccessReviewEpilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients [...] Read more.
Stigma and psychosocial problems in patients with epilepsyOpen AccessReviewEpilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients [...] Read more.Epilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients to enjoy a seizure-free life. However, throughout history, epilepsy has acquired diverse interpretations due to the experienced seizures, transforming the condition from a clinical issue into a social stigma. Therefore, the aim of this review study is to review stigma and psychosocial problems in patients with epilepsy (PwE). For this reason, this study utilises sources from the last ten years and reports current data. As a result of the review, it was found that societal discrimination in PwE arises primarily from inadequate knowledge, misconceptions, and negative attitudes toward the condition. Other contributing factors were include patients’ lower levels of education and income, frequent seizures due to inadequate treatment, age at onset, duration of the disease, depressive symptoms, and lack of social support. Also, it was found that the stigma individuals with epilepsy face plays a pivotal role in exacerbating their psychosocial problems. Unfortunately, stigma and psychosocial challenges appear to be in a vicious circle, with an increase in one increasing the other. Stigmatized patients tended to isolate themselves from society, further increasing their likelihood of experiencing a depressive mood or psychiatric comorbidity. Consequently, individuals with epilepsy encounter difficulties in various domains such as marriage, work, education, and personal life. Considering these significant psychosocial burdens, it is essential to recognize that epilepsy surpasses its medical implications. Unfortunately, current efforts to reduce stigma remain insufficient, necessitating urgent and comprehensive measures to address this issue.
Kubra YeniPublished: December 06, 2023 Explor Neurosci. 2023;2:251–263
DOI: https://doi.org/10.37349/en.2023.00026
This article belongs to the special issue EpilepsyEpilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients to enjoy a seizure-free life. However, throughout history, epilepsy has acquired diverse interpretations due to the experienced seizures, transforming the condition from a clinical issue into a social stigma. Therefore, the aim of this review study is to review stigma and psychosocial problems in patients with epilepsy (PwE). For this reason, this study utilises sources from the last ten years and reports current data. As a result of the review, it was found that societal discrimination in PwE arises primarily from inadequate knowledge, misconceptions, and negative attitudes toward the condition. Other contributing factors were include patients’ lower levels of education and income, frequent seizures due to inadequate treatment, age at onset, duration of the disease, depressive symptoms, and lack of social support. Also, it was found that the stigma individuals with epilepsy face plays a pivotal role in exacerbating their psychosocial problems. Unfortunately, stigma and psychosocial challenges appear to be in a vicious circle, with an increase in one increasing the other. Stigmatized patients tended to isolate themselves from society, further increasing their likelihood of experiencing a depressive mood or psychiatric comorbidity. Consequently, individuals with epilepsy encounter difficulties in various domains such as marriage, work, education, and personal life. Considering these significant psychosocial burdens, it is essential to recognize that epilepsy surpasses its medical implications. Unfortunately, current efforts to reduce stigma remain insufficient, necessitating urgent and comprehensive measures to address this issue.
 Impact of circadian clock dysfunction on human healthOpen AccessReviewAll living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic act [...] Read more.
Impact of circadian clock dysfunction on human healthOpen AccessReviewAll living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic act [...] Read more.All living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic activity, heart rate variability, hormone secretion, and others. The hypothalamic suprachiasmatic nucleus (SCN) acts as a primary circadian pacemaker. Peripheral tissues have an endogenous circadian clock; however, SCN synchronizes the circadian activity of the peripheral clocks. The retinohypothalamic tract (RHT) from retinal ganglionic cells carries the photic signal into the SCN that regulates the rhythmic expression of the core clock genes through the feedback loop. At the output level, the SCN connects with the pineal gland and the peripheral tissues with the help of neuroendocrine mediators. Disruption of circadian clock functions is detrimental to health. Shift work, night work, chronic or acute jet lag, and light-at-night have adverse effects on circadian functions. Misalignment of circadian rhythm alters the expression of core clock genes, leading to deregulation of cellular activity and metabolic functions. Circadian rhythm dysfunction causes many pathologic conditions, including sleep disorders, cardiovascular problems, metabolic dysfunction, infertility, poor physical performance, as well as cancer. The present work has reviewed the relationship between circadian clock dysfunction and impaired physiological activities.
Saptadip Samanta, Sk Asif AliPublished: September 29, 2022 Explor Neurosci. 2022;1:4–30
DOI: https://doi.org/10.37349/en.2022.00002
This article belongs to the special issue Circadian Rhythm and MelatoninAll living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic activity, heart rate variability, hormone secretion, and others. The hypothalamic suprachiasmatic nucleus (SCN) acts as a primary circadian pacemaker. Peripheral tissues have an endogenous circadian clock; however, SCN synchronizes the circadian activity of the peripheral clocks. The retinohypothalamic tract (RHT) from retinal ganglionic cells carries the photic signal into the SCN that regulates the rhythmic expression of the core clock genes through the feedback loop. At the output level, the SCN connects with the pineal gland and the peripheral tissues with the help of neuroendocrine mediators. Disruption of circadian clock functions is detrimental to health. Shift work, night work, chronic or acute jet lag, and light-at-night have adverse effects on circadian functions. Misalignment of circadian rhythm alters the expression of core clock genes, leading to deregulation of cellular activity and metabolic functions. Circadian rhythm dysfunction causes many pathologic conditions, including sleep disorders, cardiovascular problems, metabolic dysfunction, infertility, poor physical performance, as well as cancer. The present work has reviewed the relationship between circadian clock dysfunction and impaired physiological activities.
 Cellular and molecular mechanisms of stress-induced memory impairmentOpen AccessReviewExposure to stressful conditions plays a critical role in brain processes, including neural plasticity, synaptic transmission, and cognitive functions. Since memory-related brain regions, the hippocampus (Hip), the amygdala, and t [...] Read more.
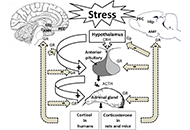
Cellular and molecular mechanisms of stress-induced memory impairmentOpen AccessReviewExposure to stressful conditions plays a critical role in brain processes, including neural plasticity, synaptic transmission, and cognitive functions. Since memory-related brain regions, the hippocampus (Hip), the amygdala, and t [...] Read more.Exposure to stressful conditions plays a critical role in brain processes, including neural plasticity, synaptic transmission, and cognitive functions. Since memory-related brain regions, the hippocampus (Hip), the amygdala, and the prefrontal cortex, express high glucocorticoid receptors (GRs), these areas are the potential targets of stress hormones. Stress affects memory encoding, consolidation, and retrieval, which may depend on many factors such as the type, duration, the intensity of the stressor or the brain region. Here, this review mainly focused on the mechanisms involved in stress-induced memory impairment. Acute/chronic stress induces structural and functional changes in neurons and glial cells. Dendritic arborization, reduction of dendritic spine density, and alteration in glutamatergic-mediated synaptic transmission via N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors are mechanisms that stress affect long-term memory formation. Exposure to acute or chronic stress could interplay with multiple neurotransmitter signaling, modulating the neuronal circuits involved in memory impairment or state-dependent learning. Stress hormones also modulate the expression of microRNAs in the specific brain regions responsible for stress-induced behaviors. Because of expressing GRs in astrocytes and microglial cells, stress could affect the morphology, structure, and functions of these glial cells in memory-related brain regions. Astrocytes play a crucial role in stress-induced aversive or fear memory formation. Over-activation of the microglial cells enhances the release of inflammatory cytokines, which results in neuronal injury. Stress has a prominent role in cognitive decline to induces memory problems, particularly in older adults. Due to the issue’s importance, here the provided overview attempted to address the question of how stress alters neuronal epigenetic regulators, synaptic transmissions, and glial activity in the brain.
Ameneh Rezayof ... Shiva HashemizadehPublished: December 30, 2022 Explor Neurosci. 2022;1:100–119
DOI: https://doi.org/10.37349/en.2022.00008
This article belongs to the special issue Neuroinflammation in the Ageing and the Injured BrainExposure to stressful conditions plays a critical role in brain processes, including neural plasticity, synaptic transmission, and cognitive functions. Since memory-related brain regions, the hippocampus (Hip), the amygdala, and the prefrontal cortex, express high glucocorticoid receptors (GRs), these areas are the potential targets of stress hormones. Stress affects memory encoding, consolidation, and retrieval, which may depend on many factors such as the type, duration, the intensity of the stressor or the brain region. Here, this review mainly focused on the mechanisms involved in stress-induced memory impairment. Acute/chronic stress induces structural and functional changes in neurons and glial cells. Dendritic arborization, reduction of dendritic spine density, and alteration in glutamatergic-mediated synaptic transmission via N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors are mechanisms that stress affect long-term memory formation. Exposure to acute or chronic stress could interplay with multiple neurotransmitter signaling, modulating the neuronal circuits involved in memory impairment or state-dependent learning. Stress hormones also modulate the expression of microRNAs in the specific brain regions responsible for stress-induced behaviors. Because of expressing GRs in astrocytes and microglial cells, stress could affect the morphology, structure, and functions of these glial cells in memory-related brain regions. Astrocytes play a crucial role in stress-induced aversive or fear memory formation. Over-activation of the microglial cells enhances the release of inflammatory cytokines, which results in neuronal injury. Stress has a prominent role in cognitive decline to induces memory problems, particularly in older adults. Due to the issue’s importance, here the provided overview attempted to address the question of how stress alters neuronal epigenetic regulators, synaptic transmissions, and glial activity in the brain.
 Negative environmental influences on the developing brain mediated by epigenetic modificationsOpen AccessReviewBrain development, a complex process, consisting of several phases, starting as early as two weeks after conception, and continuing through childhood till early adolescence, is crucial for the devel [...] Read more.
Negative environmental influences on the developing brain mediated by epigenetic modificationsOpen AccessReviewBrain development, a complex process, consisting of several phases, starting as early as two weeks after conception, and continuing through childhood till early adolescence, is crucial for the devel [...] Read more.Brain development, a complex process, consisting of several phases, starting as early as two weeks after conception, and continuing through childhood till early adolescence, is crucial for the development of properly functioning body systems, behavioral traits, and neurocognitive abilities. Infancy and childhood are recognized as important periods for initial brain formation, however in later stages of life, such as childhood and adulthood, experiences, together with environmental exposures, can still influence brain physiology. The developing brain is particularly susceptible to epigenetic changes with many factors being proposed as modifiers by directly impacting DNA methylation as well as histone and chromatin modifications within genes implicated in development. These factors include: maternal stress and diet, exposure to pollutants, sleep quality, as well as dietary habits. Evidence indicates exposures to environmental threats can lead to inappropriate neurological, metabolic, and endocrine functioning often mediated by epigenetic mechanisms with symptoms manifesting themselves as early as childhood or in later stages of life. Therefore, the main aim of this review is to evaluate the current studies focused on negative environmental exposures and their consequences on the developing brain directed by epigenetic mechanisms.
Maya Komar-Fletcher ... Joanna Michalina JurekPublished: September 28, 2023 Explor Neurosci. 2023;2:193–211
DOI: https://doi.org/10.37349/en.2023.00021Brain development, a complex process, consisting of several phases, starting as early as two weeks after conception, and continuing through childhood till early adolescence, is crucial for the development of properly functioning body systems, behavioral traits, and neurocognitive abilities. Infancy and childhood are recognized as important periods for initial brain formation, however in later stages of life, such as childhood and adulthood, experiences, together with environmental exposures, can still influence brain physiology. The developing brain is particularly susceptible to epigenetic changes with many factors being proposed as modifiers by directly impacting DNA methylation as well as histone and chromatin modifications within genes implicated in development. These factors include: maternal stress and diet, exposure to pollutants, sleep quality, as well as dietary habits. Evidence indicates exposures to environmental threats can lead to inappropriate neurological, metabolic, and endocrine functioning often mediated by epigenetic mechanisms with symptoms manifesting themselves as early as childhood or in later stages of life. Therefore, the main aim of this review is to evaluate the current studies focused on negative environmental exposures and their consequences on the developing brain directed by epigenetic mechanisms.
 Stigma and psychosocial problems in patients with epilepsyOpen AccessReviewEpilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients [...] Read more.
Stigma and psychosocial problems in patients with epilepsyOpen AccessReviewEpilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients [...] Read more.Epilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients to enjoy a seizure-free life. However, throughout history, epilepsy has acquired diverse interpretations due to the experienced seizures, transforming the condition from a clinical issue into a social stigma. Therefore, the aim of this review study is to review stigma and psychosocial problems in patients with epilepsy (PwE). For this reason, this study utilises sources from the last ten years and reports current data. As a result of the review, it was found that societal discrimination in PwE arises primarily from inadequate knowledge, misconceptions, and negative attitudes toward the condition. Other contributing factors were include patients’ lower levels of education and income, frequent seizures due to inadequate treatment, age at onset, duration of the disease, depressive symptoms, and lack of social support. Also, it was found that the stigma individuals with epilepsy face plays a pivotal role in exacerbating their psychosocial problems. Unfortunately, stigma and psychosocial challenges appear to be in a vicious circle, with an increase in one increasing the other. Stigmatized patients tended to isolate themselves from society, further increasing their likelihood of experiencing a depressive mood or psychiatric comorbidity. Consequently, individuals with epilepsy encounter difficulties in various domains such as marriage, work, education, and personal life. Considering these significant psychosocial burdens, it is essential to recognize that epilepsy surpasses its medical implications. Unfortunately, current efforts to reduce stigma remain insufficient, necessitating urgent and comprehensive measures to address this issue.
Kubra YeniPublished: December 06, 2023 Explor Neurosci. 2023;2:251–263
DOI: https://doi.org/10.37349/en.2023.00026
This article belongs to the special issue EpilepsyEpilepsy, a prevalent neurological disorder, is characterized by chronic seizures resulting from abnormal electrical activity in the brain. Adequate medical treatment allows roughly 70% of patients to enjoy a seizure-free life. However, throughout history, epilepsy has acquired diverse interpretations due to the experienced seizures, transforming the condition from a clinical issue into a social stigma. Therefore, the aim of this review study is to review stigma and psychosocial problems in patients with epilepsy (PwE). For this reason, this study utilises sources from the last ten years and reports current data. As a result of the review, it was found that societal discrimination in PwE arises primarily from inadequate knowledge, misconceptions, and negative attitudes toward the condition. Other contributing factors were include patients’ lower levels of education and income, frequent seizures due to inadequate treatment, age at onset, duration of the disease, depressive symptoms, and lack of social support. Also, it was found that the stigma individuals with epilepsy face plays a pivotal role in exacerbating their psychosocial problems. Unfortunately, stigma and psychosocial challenges appear to be in a vicious circle, with an increase in one increasing the other. Stigmatized patients tended to isolate themselves from society, further increasing their likelihood of experiencing a depressive mood or psychiatric comorbidity. Consequently, individuals with epilepsy encounter difficulties in various domains such as marriage, work, education, and personal life. Considering these significant psychosocial burdens, it is essential to recognize that epilepsy surpasses its medical implications. Unfortunately, current efforts to reduce stigma remain insufficient, necessitating urgent and comprehensive measures to address this issue.
 Neuropharmacologic modulation of the melatonergic systemOpen AccessReviewThe circadian rhythm is a critical system that governs an organism’s functions in alignment with the light-dark cycle. Melatonin release from the pineal gland plays a crucial role in regulating th [...] Read more.
Neuropharmacologic modulation of the melatonergic systemOpen AccessReviewThe circadian rhythm is a critical system that governs an organism’s functions in alignment with the light-dark cycle. Melatonin release from the pineal gland plays a crucial role in regulating th [...] Read more.The circadian rhythm is a critical system that governs an organism’s functions in alignment with the light-dark cycle. Melatonin release from the pineal gland plays a crucial role in regulating the internal clock of the body. Multiple neurotransmitter systems in the central nervous system are linked to the release of melatonin. In this review, the relationship between circadian rhythm, melatonin secretion and various neurotransmitter systems are mainly discussed. Serotonin regulates the circadian rhythm through projections from raphe nuclei. Agomelatine is an example of the synergistic interaction between melatonin and serotonin. Melatonergic agents and selective serotonin reuptake inhibitors also exert notable impacts on depression in concomitant use. Dopamine has an inhibitory effect on melatonin release, while melatonin also inhibits dopamine release. This should be taken into account when considering the use of melatonin in Parkinson’s disease. On the contrary, use of melatonin may offer therapeutic advantages for schizophrenia and tardive dyskinesia. The interaction between norepinephrine and melatonin exhibits diurnal variability, with norepinephrine promoting arousal and inhibiting daytime melatonin secretion. Melatonergic neurons also exert a specific protective influence on cholinergic neurons. Interaction between the histaminergic and melatonergic systems is significant, particularly in association with immunity, sleep, and circadian rhythm. Novel ligands with dual-acting properties, interacting with both the histaminergic and melatonergic systems are investigated. Currently, there is a limited number of approved melatonergic agents that primarily demonstrate positive effects in addressing insomnia and depression. However, there is considerable potential in studying new agents that target both the melatonergic and other neurotransmitter systems, which alleviate various conditions, including neurodegenerative diseases, dementia, autoimmune diseases, allergic diseases, epilepsy, and other neuropsychiatric disorders. The ongoing process of developing and evaluating new ligands selectively targeting the melatonergic system remains crucial in understanding the complex relationship between these systems.
Utku Aykan ... Canan UluogluPublished: December 22, 2023 Explor Neurosci. 2023;2:287–306
DOI: https://doi.org/10.37349/en.2023.00029
This article belongs to the special issue Circadian Rhythm and MelatoninThe circadian rhythm is a critical system that governs an organism’s functions in alignment with the light-dark cycle. Melatonin release from the pineal gland plays a crucial role in regulating the internal clock of the body. Multiple neurotransmitter systems in the central nervous system are linked to the release of melatonin. In this review, the relationship between circadian rhythm, melatonin secretion and various neurotransmitter systems are mainly discussed. Serotonin regulates the circadian rhythm through projections from raphe nuclei. Agomelatine is an example of the synergistic interaction between melatonin and serotonin. Melatonergic agents and selective serotonin reuptake inhibitors also exert notable impacts on depression in concomitant use. Dopamine has an inhibitory effect on melatonin release, while melatonin also inhibits dopamine release. This should be taken into account when considering the use of melatonin in Parkinson’s disease. On the contrary, use of melatonin may offer therapeutic advantages for schizophrenia and tardive dyskinesia. The interaction between norepinephrine and melatonin exhibits diurnal variability, with norepinephrine promoting arousal and inhibiting daytime melatonin secretion. Melatonergic neurons also exert a specific protective influence on cholinergic neurons. Interaction between the histaminergic and melatonergic systems is significant, particularly in association with immunity, sleep, and circadian rhythm. Novel ligands with dual-acting properties, interacting with both the histaminergic and melatonergic systems are investigated. Currently, there is a limited number of approved melatonergic agents that primarily demonstrate positive effects in addressing insomnia and depression. However, there is considerable potential in studying new agents that target both the melatonergic and other neurotransmitter systems, which alleviate various conditions, including neurodegenerative diseases, dementia, autoimmune diseases, allergic diseases, epilepsy, and other neuropsychiatric disorders. The ongoing process of developing and evaluating new ligands selectively targeting the melatonergic system remains crucial in understanding the complex relationship between these systems.
 Updates in mechanical thrombectomyOpen AccessReviewStroke is a leading cause of morbidity and mortality. The advent of mechanical thrombectomy has largely improved patient outcomes. This article reviews the features and outcomes associated with aspi [...] Read more.
Updates in mechanical thrombectomyOpen AccessReviewStroke is a leading cause of morbidity and mortality. The advent of mechanical thrombectomy has largely improved patient outcomes. This article reviews the features and outcomes associated with aspi [...] Read more.Stroke is a leading cause of morbidity and mortality. The advent of mechanical thrombectomy has largely improved patient outcomes. This article reviews the features and outcomes associated with aspiration, stent retrievers, and combination catheters used in current practice. There is also a discussion on clinical considerations based on anatomical features and clot composition. The reperfusion grading scale and outcome metrics commonly used following thrombectomy when a patient is still in the hospital are reviewed. Lastly, there are proposed discharge and outpatient follow-up goals in caring for patients hospitalized for a stroke.
Kevin Pierre ... Brandon Lucke-WoldPublished: December 30, 2022 Explor Neurosci. 2022;1:83–99
DOI: https://doi.org/10.37349/en.2022.00007Stroke is a leading cause of morbidity and mortality. The advent of mechanical thrombectomy has largely improved patient outcomes. This article reviews the features and outcomes associated with aspiration, stent retrievers, and combination catheters used in current practice. There is also a discussion on clinical considerations based on anatomical features and clot composition. The reperfusion grading scale and outcome metrics commonly used following thrombectomy when a patient is still in the hospital are reviewed. Lastly, there are proposed discharge and outpatient follow-up goals in caring for patients hospitalized for a stroke.
 Impact of circadian clock dysfunction on human healthOpen AccessReviewAll living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic act [...] Read more.
Impact of circadian clock dysfunction on human healthOpen AccessReviewAll living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic act [...] Read more.All living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic activity, heart rate variability, hormone secretion, and others. The hypothalamic suprachiasmatic nucleus (SCN) acts as a primary circadian pacemaker. Peripheral tissues have an endogenous circadian clock; however, SCN synchronizes the circadian activity of the peripheral clocks. The retinohypothalamic tract (RHT) from retinal ganglionic cells carries the photic signal into the SCN that regulates the rhythmic expression of the core clock genes through the feedback loop. At the output level, the SCN connects with the pineal gland and the peripheral tissues with the help of neuroendocrine mediators. Disruption of circadian clock functions is detrimental to health. Shift work, night work, chronic or acute jet lag, and light-at-night have adverse effects on circadian functions. Misalignment of circadian rhythm alters the expression of core clock genes, leading to deregulation of cellular activity and metabolic functions. Circadian rhythm dysfunction causes many pathologic conditions, including sleep disorders, cardiovascular problems, metabolic dysfunction, infertility, poor physical performance, as well as cancer. The present work has reviewed the relationship between circadian clock dysfunction and impaired physiological activities.
Saptadip Samanta, Sk Asif AliPublished: September 29, 2022 Explor Neurosci. 2022;1:4–30
DOI: https://doi.org/10.37349/en.2022.00002
This article belongs to the special issue Circadian Rhythm and MelatoninAll living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic activity, heart rate variability, hormone secretion, and others. The hypothalamic suprachiasmatic nucleus (SCN) acts as a primary circadian pacemaker. Peripheral tissues have an endogenous circadian clock; however, SCN synchronizes the circadian activity of the peripheral clocks. The retinohypothalamic tract (RHT) from retinal ganglionic cells carries the photic signal into the SCN that regulates the rhythmic expression of the core clock genes through the feedback loop. At the output level, the SCN connects with the pineal gland and the peripheral tissues with the help of neuroendocrine mediators. Disruption of circadian clock functions is detrimental to health. Shift work, night work, chronic or acute jet lag, and light-at-night have adverse effects on circadian functions. Misalignment of circadian rhythm alters the expression of core clock genes, leading to deregulation of cellular activity and metabolic functions. Circadian rhythm dysfunction causes many pathologic conditions, including sleep disorders, cardiovascular problems, metabolic dysfunction, infertility, poor physical performance, as well as cancer. The present work has reviewed the relationship between circadian clock dysfunction and impaired physiological activities.
 Update for astrocytomas: medical and surgical management considerationsOpen AccessReviewAstrocytomas include a wide range of tumors with unique mutations and varying grades of malignancy. These tumors all originate from the astrocyte, a star-shaped glial cell that plays a major role in [...] Read more.
Update for astrocytomas: medical and surgical management considerationsOpen AccessReviewAstrocytomas include a wide range of tumors with unique mutations and varying grades of malignancy. These tumors all originate from the astrocyte, a star-shaped glial cell that plays a major role in [...] Read more.Astrocytomas include a wide range of tumors with unique mutations and varying grades of malignancy. These tumors all originate from the astrocyte, a star-shaped glial cell that plays a major role in supporting functions of the central nervous system (CNS), including blood-brain barrier (BBB) development and maintenance, water and ion regulation, influencing neuronal synaptogenesis, and stimulating the immunological response. In terms of epidemiology, glioblastoma (GB), the most common and malignant astrocytoma, generally occur with higher rates in Australia, Western Europe, and Canada, with the lowest rates in Southeast Asia. Additionally, significantly higher rates of GB are observed in males and non-Hispanic whites. It has been suggested that higher levels of testosterone observed in biological males may account for the increased rates of GB. Hereditary syndromes such as Cowden, Lynch, Turcot, Li-Fraumeni, and neurofibromatosis type 1 have been linked to increased rates of astrocytoma development. While there are a number of specific gene mutations that may influence malignancy or be targeted in astrocytoma treatment, O6-methylguanine-DNA methyltransferase (MGMT) gene function is an important predictor of astrocytoma response to chemotherapeutic agent temozolomide (TMZ). TMZ for primary and bevacizumab in the setting of recurrent tumor formation are two of the main chemotherapeutic agents currently approved in the treatment of astrocytomas. While stereotactic radiosurgery (SRS) has debatable implications for increased survival in comparison to whole-brain radiotherapy (WBRT), SRS demonstrates increased precision with reduced radiation toxicity. When considering surgical resection of astrocytoma, the extent of resection (EoR) is taken into consideration. Subtotal resection (STR) spares the margins of the T1 enhanced magnetic resonance imaging (MRI) region, gross total resection (GTR) includes the margins, and supramaximal resection (SMR) extends beyond the margin of the T1 and into the T2 region. Surgical resection, radiation, and chemotherapy are integral components of astrocytoma treatment.
Hereditary risk factors, genetic mutations, and imaging modalities are discussed in reference to astrocytoma staging and mechanism of growth. In terms of the treatment of astrocytomas, chemotherapy, radiation therapy, and strategic surgical interventions are discussed
Matthew Willman ... Brandon Lucke-WoldPublished: February 23, 2023 Explor Neurosci. 2023;2:1–26
DOI: https://doi.org/10.37349/en.2023.00009Astrocytomas include a wide range of tumors with unique mutations and varying grades of malignancy. These tumors all originate from the astrocyte, a star-shaped glial cell that plays a major role in supporting functions of the central nervous system (CNS), including blood-brain barrier (BBB) development and maintenance, water and ion regulation, influencing neuronal synaptogenesis, and stimulating the immunological response. In terms of epidemiology, glioblastoma (GB), the most common and malignant astrocytoma, generally occur with higher rates in Australia, Western Europe, and Canada, with the lowest rates in Southeast Asia. Additionally, significantly higher rates of GB are observed in males and non-Hispanic whites. It has been suggested that higher levels of testosterone observed in biological males may account for the increased rates of GB. Hereditary syndromes such as Cowden, Lynch, Turcot, Li-Fraumeni, and neurofibromatosis type 1 have been linked to increased rates of astrocytoma development. While there are a number of specific gene mutations that may influence malignancy or be targeted in astrocytoma treatment, O6-methylguanine-DNA methyltransferase (MGMT) gene function is an important predictor of astrocytoma response to chemotherapeutic agent temozolomide (TMZ). TMZ for primary and bevacizumab in the setting of recurrent tumor formation are two of the main chemotherapeutic agents currently approved in the treatment of astrocytomas. While stereotactic radiosurgery (SRS) has debatable implications for increased survival in comparison to whole-brain radiotherapy (WBRT), SRS demonstrates increased precision with reduced radiation toxicity. When considering surgical resection of astrocytoma, the extent of resection (EoR) is taken into consideration. Subtotal resection (STR) spares the margins of the T1 enhanced magnetic resonance imaging (MRI) region, gross total resection (GTR) includes the margins, and supramaximal resection (SMR) extends beyond the margin of the T1 and into the T2 region. Surgical resection, radiation, and chemotherapy are integral components of astrocytoma treatment.
Hereditary risk factors, genetic mutations, and imaging modalities are discussed in reference to astrocytoma staging and mechanism of growth. In terms of the treatment of astrocytomas, chemotherapy, radiation therapy, and strategic surgical interventions are discussed
 Therapeutic potential of extracellular vesicles in Parkinson’s diseaseOpen AccessReviewGlobally, the incidence of Parkinson’s disease (PD) is increasing faster than other neurodegenerative disorders. Neuropathologically, PD is characterized by the loss of dopaminergic neurons in the [...] Read more.
Therapeutic potential of extracellular vesicles in Parkinson’s diseaseOpen AccessReviewGlobally, the incidence of Parkinson’s disease (PD) is increasing faster than other neurodegenerative disorders. Neuropathologically, PD is characterized by the loss of dopaminergic neurons in the [...] Read more.Globally, the incidence of Parkinson’s disease (PD) is increasing faster than other neurodegenerative disorders. Neuropathologically, PD is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta due to the accumulation of aggregates of misfolded α-synuclein (α-Syn) in the cytoplasm of these neurons, forming Lewy bodies. Extracellular vesicles (EVs) are associated with the spread of α-Syn to different brain areas. However, at the same time that these EVs contribute to the pathophysiology of PD, they can also be explored as therapeutic, serving as a vehicle to deliver specific molecules, since these vesicles can easily cross the blood-brain barrier. Thus, this review summarizes the recent progress in EVs as a therapeutic strategy for PD, focusing on their delivery to the brain, and discusses the potential challenges and future directions in this field.
Michelli Ramires Teixeira ... Rodrigo Pinheiro AraldiPublished: June 29, 2023 Explor Neurosci. 2023;2:106–122
DOI: https://doi.org/10.37349/en.2023.00016
This article belongs to the special issue Extracellular Vesicles as Cell-based TherapeuticsGlobally, the incidence of Parkinson’s disease (PD) is increasing faster than other neurodegenerative disorders. Neuropathologically, PD is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta due to the accumulation of aggregates of misfolded α-synuclein (α-Syn) in the cytoplasm of these neurons, forming Lewy bodies. Extracellular vesicles (EVs) are associated with the spread of α-Syn to different brain areas. However, at the same time that these EVs contribute to the pathophysiology of PD, they can also be explored as therapeutic, serving as a vehicle to deliver specific molecules, since these vesicles can easily cross the blood-brain barrier. Thus, this review summarizes the recent progress in EVs as a therapeutic strategy for PD, focusing on their delivery to the brain, and discusses the potential challenges and future directions in this field.
Special Issues Medicinal Plants and Bioactive Phytochemicals in Neuroprotection
Medicinal Plants and Bioactive Phytochemicals in NeuroprotectionProf. Marcello Iriti
Submission Deadline: June 30, 2025
Published Articles: 3
 Novel Therapeutic Approaches for the Treatment of Depression
Novel Therapeutic Approaches for the Treatment of DepressionProf. Dirk M. Hermann Dr. Ayan Mohamud Yusuf
Submission Deadline: June 20, 2024
Published Articles: 6
 Cerebral Ischemia, Genetics, Comorbidities, Risk Factors and New Therapeutic Options for Neurorestoration
Cerebral Ischemia, Genetics, Comorbidities, Risk Factors and New Therapeutic Options for NeurorestorationProf. Aurel Popa-Wagner
Submission Deadline: March 31, 2025
Published Articles: 5
 Circadian Rhythm and Melatonin
Circadian Rhythm and MelatoninProf. Ertugrul Kilic
Submission Deadline: June 30, 2024
Published Articles: 7
 Neuroinflammation in the Ageing and the Injured Brain
Neuroinflammation in the Ageing and the Injured BrainProf. Ameneh Rezayof Dr. Maryam Sardari
Submission Deadline: June 30, 2025
Published Articles: 3
 Extracellular Vesicles as Cell-based Therapeutics
Extracellular Vesicles as Cell-based TherapeuticsProf. Dirk M. Hermann Dr. Chen Wang
Submission Deadline: June 30, 2025
Published Articles: 3
From the Editor-in-Chief It is a great pleasure to serve as the newly appointed Editor-in-Chief of Exploration of Neuroscience (EN). EN will be a strong platform for the publication of groundshaking, cutting edge science that will expand our knowledge of neurological diseases and disease treatment. Advances in molecular, cellular and system neurosciences have greatly expanded our understanding of the healthy and the injured brain in recent years, which has set up fascinating perspectives for the development of new treatment options. With the infrastructures and support of Open Exploration Publishing services, the new EN journal seeks to become a reference in discovery and education in neurological diseases.Dirk M. Hermann
It is a great pleasure to serve as the newly appointed Editor-in-Chief of Exploration of Neuroscience (EN). EN will be a strong platform for the publication of groundshaking, cutting edge science that will expand our knowledge of neurological diseases and disease treatment. Advances in molecular, cellular and system neurosciences have greatly expanded our understanding of the healthy and the injured brain in recent years, which has set up fascinating perspectives for the development of new treatment options. With the infrastructures and support of Open Exploration Publishing services, the new EN journal seeks to become a reference in discovery and education in neurological diseases.Dirk M. Hermann
Editor-in-Chief of Exploration of Neuroscience -
-
Newsletter
-
Selected Special Issues