Affiliation:
1Department of Vascular and Endovascular Surgery, Faculty of Medicine, Mansoura University, Mansoura 35111, Egypt
Email: Soliman_mosaad@hotmail.com
ORCID: https://orcid.org/0000-0001-7171-8165
Affiliation:
1Department of Vascular and Endovascular Surgery, Faculty of Medicine, Mansoura University, Mansoura 35111, Egypt
ORCID: https://orcid.org/0000-0002-0253-8395
Affiliation:
1Department of Vascular and Endovascular Surgery, Faculty of Medicine, Mansoura University, Mansoura 35111, Egypt
ORCID: https://orcid.org/0000-0002-7774-3350
Affiliation:
2Department of Radiology, Faculty of Medicine, Mansoura University, Mansoura 35111, Egypt
ORCID: https://orcid.org/0009-0005-9714-1372
Affiliation:
1Department of Vascular and Endovascular Surgery, Faculty of Medicine, Mansoura University, Mansoura 35111, Egypt
ORCID: https://orcid.org/0000-0002-8022-5589
Explor Neurosci. 2023;2:153–159 DOI: https://doi.org/10.37349/en.2023.00019
Received: March 12, 2023 Accepted: July 13, 2023 Published: August 31, 2023
Academic Editor: Suyue Pan, Southern Medical University, China
Several bare metals, self-expanding stents have been approved by the Food and Drug Administration (FDA) to treat carotid stenosis, but no covered stents have been particularly examined or approved for carotid or cerebrovascular applications. Nonetheless, there are a number of potentially useful applications for covered stents in the brachiocephalic, carotid, and even intracranial arteries. As with currently accepted applications for bare metal carotid stents, the use of covered stents in carotid arteries has been reserved for patients who are at high risk for complications with open surgical management of their specific problem. The present case report emphasizes the safety and efficacy of covered stent in complex carotid artery reconstruction entailing stenosis and aneurysmal dilatation and through light on its impact on minimizing the risk of ischemic complications associated with endovascular or surgical carotid sacrifice.
Atherosclerotic disease of the carotid arteries occurs in 10–15% of stroke patients, which contributes significantly to morbidity and mortality [1]. Once a patient with clinically substantial carotid stenosis has been diagnosed, the right course of treatment must be established, with the main goal of lowering the risk of stroke. When patients have significant symptoms of carotid artery disease or when all other medical treatments have failed, surgical intervention with carotid endarterectomy (CEA) has been found to lower the risk of ischemic stroke in the future [2].
Endovascular techniques’ novel options have sparked a sector of medical devices that is rapidly developing and enables safe, frequently less invasive substitutes for carotid revascularization. In certain circumstances, carotid artery stenting (CAS) is now a commonly acknowledged substitute for endarterectomy. The current guidelines of CAS support its use in symptomatic patients with high-grade stenosis who have deemed too high a medical risk to undergo open surgery or in lower medical risk patients with higher anatomic risk factors for CEA such as inaccessible lesion location, restenosis, or neck radiation [3].
Technologies for CAS are evolving rapidly. Stent technology facets include free-cell area, bare-metal versus coated stents, and stent tapering. Self-expanding stents have become the standard in the treatment of carotid bifurcation disease because of the significant chronic compressive forces, significant diameter disparity, and movable nature of the common and internal carotid arteries [4]. The novel use of MicroNet-covered stent has remarkably reduced periprocedural as well as postprocedural cerebral embolism compared to the conventional carotid stent [5].
The wide variety of applications of the covered stent in other vessels is also applicable in carotid arteries, including aneurysms, pseudoaneurysms, dissection, traumatic injuries, and in-stent restenosis. Although there isn’t a covered stent on the market that has Food and Drug Administration (FDA) approval for carotid artery applications, numerous alternative devices have been used, according to published reports [6]. Although no covered stents have been specifically investigated or approved for carotid or cerebrovascular applications, however, several bare metal and self-expanding stents have been approved by the FDA to treat carotid stenosis. Nevertheless, coated stents have several potential applications in the brachiocephalic, carotid, and even cerebral arteries. Patients who are at high risk of open surgical repair are candidates for both covered stents and bare metal stents. The present case report throws light on the safety and efficacy of covered stent in complex carotid artery reconstruction entailing stenosis and aneurysmal dilatation and emphasize its impact on minimizing the risk of ischemic complications associated with endovascular or surgical carotid sacrifice.
A 65-year-old diabetic, smoker male patient suffering from ischemic heart disease (IHD) was prepared for coronary artery bypass graft (CABG). Doppler ultrasonography (US) as a part of routine preoperative carotid workup revealed fibrofatty, eccentric short plaque-causing more than 70% stenosis of the proximal part of the internal carotid artery (ICA) with post stenotic aneurysmal dilatation. Findings were confirmed by computed tomography (CT) angiography (CTA) (Figure 1). A basal CT brain did not demonstrate acute or established cerebral infarction.
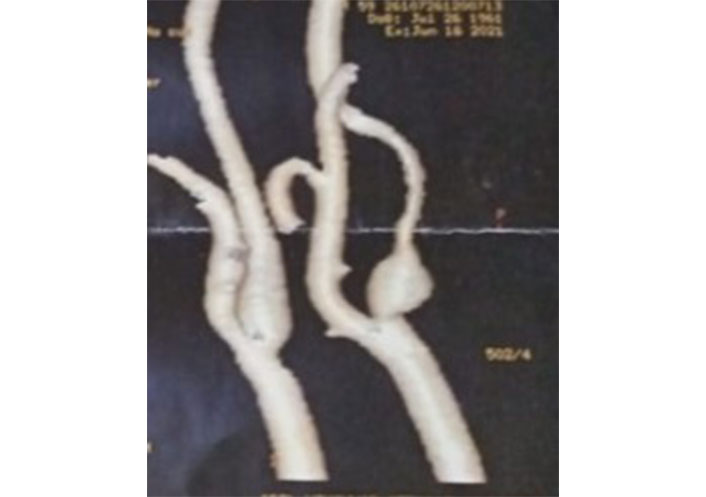
CTA of the carotid arteries showing focal stenosis of the ICA with post-stenotic dilatation and distal attenuation
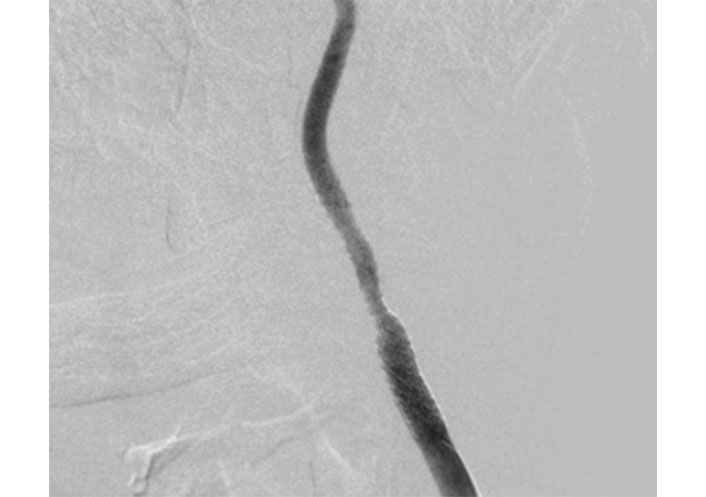
In case there is CEA morbidity due to the coronary condition and the requirement for general anesthesia, CAS was chosen with a pre-operative clopidogrel loading dosage (300 mg) administered. Selective catheterization of the left common carotid artery (CCA) was carried out under local anesthetic utilizing a 5 French Sidewinder catheter (SIM1 Tempo, Cordis, Buckinghamshire, UK) and a hydrophilic 0.035-inch guidewire via femoral endovascular access (Figure 2). Diagnostic angiography confirms the left CCA and ICA stenosis consistent with US and CTA. Heparin (5,000 units i.v.) was given.
Lateral and anteroposterior angiography were taken to quantify cerebral blood flow. The guidewire’s tip was used to bridge the lesion under the guidance of digital road mapping before being inserted into the distal ICA. Advanta V12 Covered [externally supported polytetrafluoroethylene (ePTFE) polymer coated] balloon-expandable stent graft 6 mm × 60 mm was deployed to cover the area extending from the distally attenuated segment to the common carotid isolating the stenosis, post-stenotic dilatation, and the distal attenuation segment.
To match the inner lumen of the carotid artery’s contour and address the diameter difference between the common and internal carotid arteries, the stent graft was purposefully tapered (Figure 3). The baseline diameter of CCA was 6 mm, so the stent-graft customization was achieved by post-dilatation of the distal part of the stent graft (inside ICA) to 4 mm, dilation of stenosis to 5 mm, and dilation of the proximal part of the stent-graft (inside CCA) to 7 mm.
The procedure was performed without cerebral protection or pre-dilation. Final angiography showed an excellent morphologic result with the intentional tapering of the stent-graft that successfully excluded the aneurysm and maintained good forward flow into the distal ICA and circle of Willis (Figure 4) and cerebral angiogram revealed clear cerebral vasculature (Figure 5). The patient was given a therapeutic low molecular weight heparin for 24 h.
Final angiography with the successful exclusion of the aneurysm, correction of stenosis, and distal attenuation
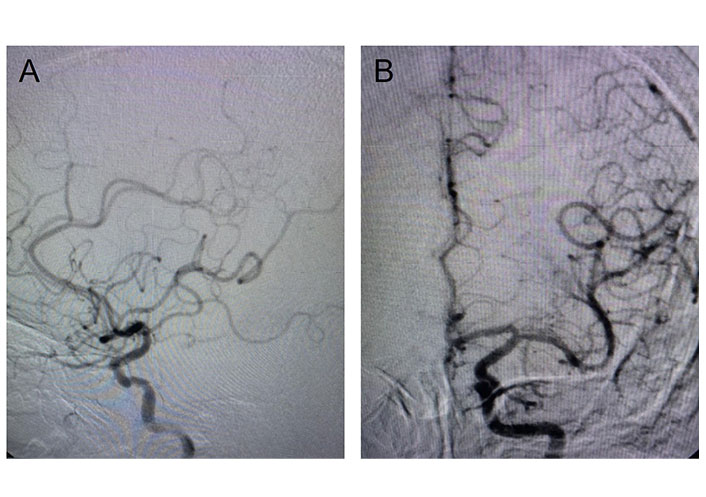
Cerebral angiogram. A. Post-stenting cerebral angiogram shows clear cerebral vasculature; B. post-stenting cerebral angiogram shows a normal anterior circle of Willis
One day later, a brain CT was done. No new ischemic lesions appeared. Without experiencing any neurological issues, the patient was released. The patient had no neurological deficit or technical issues at the one-month follow-up. CTA after two months revealed no signs of aneurysm re-filling, providing excellent stent graft flow.
The rationale behind implanting a covered stent is that it might stop the material from soft lesions and thrombus from embolizing to the distal circulation, which is how atherosclerotic carotid stenosis is treated. By forcing the plaque against the vascular wall in some individuals, it may also prevent plaque material from prolapsing between the stent struts and into the lumen, so preventing micro-embolization. According to some researchers, exposed atherosclerotic surfaces in bare-metal stents with inadequate luminal wall coverage increase the risk of peri-procedural embolic events [4]. Intrastent protrusion (ISP) correlates with the occurrence of vulnerable plaques and debris capture. Nonetheless, it can be fixed with stent-in-stent placement [7].
With the advent of cerebral protection devices, the rate of peri-procedural ischemic complications has substantially lowered [5, 7]. The fragmented plaque particles may embolize, causing symptomatic neurologic complications as well as asymptomatic brain lesions seen on diffusion-weighted magnetic resonance imaging (MRI) [8]. Therefore, the idea of coating the plaque with a covered stent, to reduce fragmentation and distal embolization of the plaque particles, in almost straight vessels made a paradigm shift in the armamentarium of management of carotid artery lesions.
The simplicity and cost-effectiveness of covered stent implantation without distal protection are the main advantages compared with stent placement with protection. Moreover, flexible-covered stents [9] or Willis-covered stents [10] may again be considered an option in patients with extremely tortuous cervical ICA that interferes with the safe deployment of a filter without inducing vasospasm.
The risk of peri-procedural stroke extends from 24 h to 30 days after the procedure. This supports the suggestion that perioperative stroke occurring as a complication of CAS is not solely a technical problem. Although the cause of late stroke after CAS is unexplained, plaque protrusion through the struts may be one of the potential causes. Shinozaki et al. [11] observed that the frequency of plaque protrusion into the stent in CAS patients was 7.8% by the intravascular ultrasound (IVUS) and proved superior to CTA and magnetic resonance angiography (MRA) in stent selection [12].
In the current case, plaque protrusion, aneurysmal connection, and distal attenuation were alleviated altogether by using a covered stent, moreover, it seems intuitive to avoid the bare metal stent because of the possibility of plaque protrusion lack of aneurysm coverage and insufficient radial force to support the attenuated ICA.
In conclusion, the efforts to select the ideal stent for atherosclerotic carotid lesions are in progress. Covered stents offer a fast and simple procedure, especially in patients with anatomical tortuosity in the cervical ICA that precludes safe deployment of filter devices. Irrespective of the lack of data discussing their use, prevention of distal embolization seems possible by compressing the plaque against the vessel wall and isolating it from circulation using stent grafts.
CAS: carotid artery stenting
CCA: common carotid artery
CEA: carotid endarterectomy
CT: computed tomography
CTA: computed tomography angiography
ICA: internal carotid artery
MS: Conceptualization, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing. KM, MAE, Rosan S and Reem S: Writing—review & editing. All authors contributed to the study conception and design, commented on previous versions, read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
All of the procedure details whether diagnostic or therapeutic as well as the publication of the data were approved by the patient via written consent and approved by IRB (code: R.22.09.1805.R1.R2-202212-6), and all the procedures were done in compliance with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from participants.
Informed consent to publication was obtained from relevant participants.
The raw data supporting the conclusions of this manuscript will be made available by the authors (Soliman_mosaad@hotmail.com), without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3164
Download: 32
Times Cited: 0
