Affiliation:
Department of Physiology, Midnapore College, Midnapore, Paschim Medinipur 721101, West Bengal, India
Email: saptadip174@gmail.com; saptadip.samanta@midnaporecollege.ac.in
ORCID: https://orcid.org/0000-0001-6741-699X
Affiliation:
Department of Physiology, Midnapore College, Midnapore, Paschim Medinipur 721101, West Bengal, India
ORCID: https://orcid.org/0000-0003-0505-7375
Explor Neurosci. 2022;1:4–30 DOI: https://doi.org/10.37349/en.2022.00002
Received: March 03, 2022 Accepted: June 27, 2022 Published: September 29, 2022
Academic Editor: Ertugrul Kilic, Istanbul Medipol University, Turkey
The article belongs to the special issue Circadian Rhythm and Melatonin
All living organisms exhibit circadian rhythms. Humans show circadian rhythm of the different physiological functions such as sleep-wake cycle, core body temperature, feeding behavior, metabolic activity, heart rate variability, hormone secretion, and others. The hypothalamic suprachiasmatic nucleus (SCN) acts as a primary circadian pacemaker. Peripheral tissues have an endogenous circadian clock; however, SCN synchronizes the circadian activity of the peripheral clocks. The retinohypothalamic tract (RHT) from retinal ganglionic cells carries the photic signal into the SCN that regulates the rhythmic expression of the core clock genes through the feedback loop. At the output level, the SCN connects with the pineal gland and the peripheral tissues with the help of neuroendocrine mediators. Disruption of circadian clock functions is detrimental to health. Shift work, night work, chronic or acute jet lag, and light-at-night have adverse effects on circadian functions. Misalignment of circadian rhythm alters the expression of core clock genes, leading to deregulation of cellular activity and metabolic functions. Circadian rhythm dysfunction causes many pathologic conditions, including sleep disorders, cardiovascular problems, metabolic dysfunction, infertility, poor physical performance, as well as cancer. The present work has reviewed the relationship between circadian clock dysfunction and impaired physiological activities.
Time is an everyday phenomenon of the earth’s rotation on its axis. It can be measured but not seen or touched and weighted. Rotation of the Earth governs the natural period along with the daily light-dark (LD) cycle and other environmental phenomena like ambient temperature, tide, etc. Many biochemical and physiological functions of the living organisms rhythmically oscillate under constant environmental conditions for a period of close to 24 h. These rhythmic phenomena are called circadian rhythms [1, 2]. The circadian rhythm is present in all living organisms, from bacteria to humans. The term circadian was coined by Halberg [3]. It originated from the Latin words circa means “around” and dies means “day”. Human shows several rhythmic phenomena like a sleep-wake cycle, core body temperature, heart rate, blood pressure, feeding pattern, metabolism, hormone secretion, immune responses, and neuronal functions [4–9].
The suprachiasmatic nucleus (SCN) of the anterior hypothalamus performs the functions of the central clock and regulates the oscillations of physiological rhythms. Non-visual retinal ganglionic cells transmit photic signals to the SCN through the RHT after exposure to light detected by the retina [10, 11]. The photic signal controls the functions of SCN by regulating the expression of clock genes. More specifically, photic signals lead to transient up-regulation of Period 1 (Per1) and Per2 expression in the SCN. SCN also synchronizes the circadian oscillations of peripheral tissue clocks through neuro-endocrinal factors [12, 13].
Disruption of the circadian pattern is a common incident in our modern society. Chronic and acute jet lag, shift works, night works, and extended shifts are the causes of circadian dysfunction that potentially enhances sleep disorders, poor physical performance, metabolic syndrome, cardiovascular and inflammatory diseases, neuropsychiatric illness, and cancer [14–16]. The present review has focused on the impact of circadian rhythm dysfunction on different physiological functions.
SCN acts as a master clock in the mammalian brain [17, 18]. The SCN is a paired structure, bilaterally situated on either side of the third ventricle just above the optic chiasm [10, 18]. The neurons of the SCN are the smallest in size (~10 μm) and are closely packed in the core and shell region. The nuclei of SCN get photic signals through the RHT after exposure to light on the retina [10, 11]. Melanopsin (OPN4) containing non-visual retinal ganglion cells (RGCs) are most sensitive to blue light (464–484 nm wavelengths) and transmit the impulse to the SCN via RHT [19, 20]. RHT starts the signal transduction process in the SCN and ultimately regulates the expression of several clock genes. Additionally, peripheral tissues also express clock genes. RHT is a glutamatergic pathway. Glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP) act as the primary neurotransmitters. Glutamate acts through α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors. Activation of glutamatergic receptors increases intracellular calcium that activates different kinases such as calcium/calmodulin-dependent protein kinase (CaMK), mitogen-activated protein kinase (MAPK), and protein kinase A (PKA). The subsequent result is phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding protein (CREB). Phosphorylated CREB (p-CREB) then translocates to the nucleus and binds with calcium/cAMP response elements (CREs) in association with co-activators for the regulation of gene expression [18, 21–23]. Tischkau et al. [23] reported that the 5’-flanking region of Per1 and Per2 genes carried CRE that interacts with CREB for the expression of Per genes. The mutational study indicated the role of CRE in the expression of Per genes [17]. On the other hand, output signals from SCN reach the peripheral organs for the regulation of physiological and behavioral functions [24].
SCN makes a complex connection with the peripheral tissue clocks through the neural and hormonal mediators for the synchronization of circadian oscillations [12, 13]. Despite SCN-mediated regulation, all cells of the body have an endogenous clock system that can function autonomously to maintain circadian oscillations. At the molecular level, the activity of the circadian clock is driven by a complex network of transcription-translation feedback loop that regulates the rhythmic expression of clock-related genes. Transcription factors circadian locomotor output cycles kaput (CLOCK) and brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (BMAL1) induce the expression of core clock components, Per and cryptochrome (Cry). Subsequently, they regulate the activity of CLOCK and BMAL1 [25]. The output signals from the SCN neurons are associated with the expression of clock-controlled genes (CCGs). These genes contain promoter sequence (CACGTG) for binding of CLOCK-BMAL1 complex (E-box) in their upstream for the operation of the transcriptional cascade. The promoter region of arginine vasopressin (Avp), D-element binding protein (Dbp), and prokineticin 2 (Pk2) contain E-box. AVP acts as a neurotransmitter, DBP is a transcription factor, and PK2 is a regulator of different biological functions (sleep cycle, behavioral rhythm, and others) [26]. The endogenous clock system is synchronized with several environmental time cues (zeitgebers) like temperature, LD cycle, physical activity, and feeding behavior [27–29]. The external zeitgebers regulate the expression of the molecular components of the endogenous clock [26].
Approximately 12–14 core clock genes are most important for the regulation of human circadian rhythm [6, 30, 31]. The complex transcriptional/translational feedback loops (TTFLs) control the overall expression of core clock genes (Figure 1) [32, 33]. The common core clock genes are 3 Per genes (Per1–3), 2 Cry genes (Cry1–2), Bmal1, Clock, 3 retinoid-related orphan receptor (Ror, Ror α, β, and γ) genes, 2 genes of reverse-erythroblastosis (Rev-Erb; Rev-Erb α and β). A specific enzyme casein kinase 1 (CK1; CK1δ and ε) is a part of the mammalian circadian system. Furthermore, some other genes [neuronal period-aryl hydrocarbon receptor nuclear translocator single-minded protein 2 (Npas2/Mop4), Aryl hydrocarbon receptor nuclear translocator-like protein 2 (Arntl2/Mop9), and F-box/LRR repeat protein 3 (Fbxl3)] are also associated with clock functions.
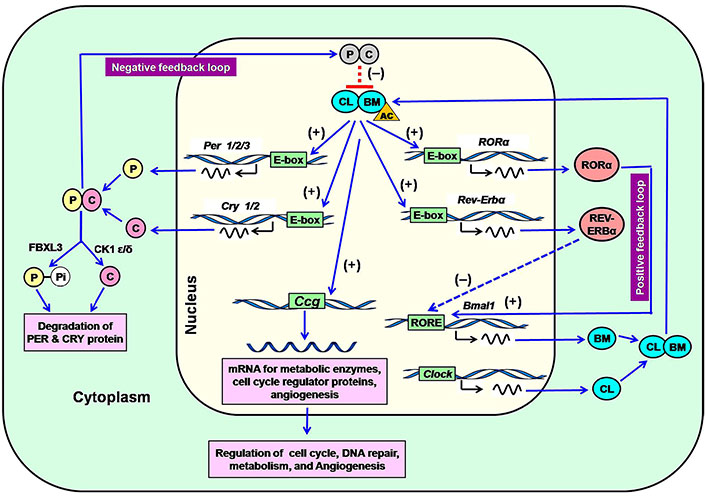
Regulation of expression of core clock genes in the SCN through the positive and negative feedback loop in the mammalian circadian system. P: PER; C: CRY; (-): repression; (+): induction; CL: CLOCK; BM: BMAL1; AC: acetyl group; Pi: phosphate; RORE: Ror response element; FBXL3: F-box protein, E3 ligase; Ccg: clock-controlled genes
Note. Adapted from “Melatonin: an endogenous miraculous indolamine, fights against cancer progression,” by Samanta S. J Cancer Res Clin Oncol. 2020;146:1893–922 (https://doi.org/10.1007/s00432-020-03292-w). © Springer-Verlag GmbH Germany, part of Springer Nature 2020.
The expression of Per, Cry, Ror α, and Rev-Erb α is mediated by the CLOCK-BMAL1 complex (Figure 1). CLOCK-BMAL1 heterodimer initially influences the rhythmic expression of the Per and Cry genes after binding at the E-box. Translation of PER and CRY in the cytoplasm promotes their accumulation and forms a heterodimer. Heterodimeric PER and CRY move into the nucleus and inhibit the transactivating effects of the CLOCK-BMAL1 complex, leading to the termination of transcription of Per, Cry, Ror, and Rev-Erb genes (Figure 1). This inhibition completes the negative feedback effects (Figure 1) [34, 35]. CRY and PER concentrations decrease at night, resulting in the discontinuation of their repressive activity. This event starts a new cycle of CLOCK-BMAL1 complex-mediated transcriptional activation [6, 36]. Cytoplasmic CKs [CK1δ and CK1ε] and F-box protein (FbxL3) are involved in the degradation of PER and CRY. CK1δ and CK1ε cleave the PER proteins after phosphorylation and ubiquitination [37, 38]. The ubiquitin E3 ligase (F-box protein), called FbxL3 degrades CRYs [39].
The positive feedback loop starts when CLOCK and BMAL1 induce the expression of other clock genes. CLOCK-BMAL1 complex recognizes E-box, which bears a specific nucleotide sequence CACGTG at the upstream of the core clock genes [40, 41]. This interaction exerts inductive effects on the expression of core clock genes. The orphan nuclear receptors Ror α and Rev-Erb α regulate the positive feedback loop. They translocate to the nucleus from the cytoplasm and recognize the RORE that contains a specific sequence (AAAGTAGGTCA) upstream of the Bmal1 promoter. Ror α induces the expression of Bmal1, while Rev-Erb α represses Bmal1 transcription. This antagonistic effect controls the rhythmic expression of Bmal1 [28, 34, 41]. The mutation of the Clock gene significantly downregulates the expression of the Per1–3 and Cry1–2 levels in SCN [42, 43]. In addition to CLOCK, NPAS2 (MOP4) can form a heterodimer with BMAL1 in the SCN to regulate the transcriptional activity. The expression of NPAS2 commonly occurs in peripheral vascularized tissues. The peripheral cells remain rhythmic in the absence of CLOCK; however, Npas2 knockout cells exhibit arrhythmic behavior [44].
The circadian oscillators also exist in individual organs and cells. The SCN is the mediator of synchronization for the other oscillators, including different parts of the brain and peripheral organs. This synchronization shows a period that is closer to 24 h. However, there is a phase delay relative to SCN in peripheral tissues. Large phase delay between SCN and peripheral tissues has been observed in nocturnal animals compared to diurnal species. Mure et al. [45] compared the expression of core clock components in SCN and different tissues of baboons and mice. They observed that there was ~12 h phase delay in the peak expression of clock components in the peripheral tissues of mice and baboons. The peak expression of Bmal1 and Per1 in the baboons occurred in the evening and morning, respectively, whereas, mice showed a reverse expression pattern (peak phase in morning and evening, respectively). However, Cry1 showed ~7 h phase delay compared to Bmal1 and Per1. The phase delay of other clock components in baboons and mice showed a similar pattern of Bmal1 and Per1. On the other hand, the expression pattern of clock components in SCN was not strictly in the opposite fashion. The peak phase of Bmal1 occurred at the same time (evening) in mice and baboons. However, phase delay was observed in the expression of Per1 and Cry2. Thus, there was a large phase delay in the peak expression of clock components in peripheral tissues between nocturnal and diurnal species.
The process of synchronization between SCN and peripheral tissues is driven by the autonomic nervous system and hormones. However, circadian modulation of body temperature and feeding behavior have a less direct effect [18]. Generally, Per genes express in the brain and other peripheral tissues such as the liver and skeletal muscle [46]. The oscillation of each Per gene in the peripheral tissue of mammals synchronizes with SCN. The phase relationship between peripheral organs and SCN is linked with LD cycles [47]. Yamazaki et al. [48] reported that the absence of SCN in transgenic rats showed gradual resetting of rhythms in different tissues. A study on genetically ablated SCN indicated that the LD cycle controls circadian rhythmicity in peripheral tissues [49]. Thus, peripheral clocks can independently oscillate with the LD cycle without the influence of SCN. The SCN and endogenous zeitgeber (melatonin) regulate the autonomic nervous system, neuroendocrine secretion, body temperature, and sleep-wake cycle. However, food acts as a potent zeitgeber and effectively affects peripheral oscillation [50]. The activity of the liver synchronizes with signals derived from feeding [51–53]. The metabolites of carbohydrate, lipid, amino acids, nucleotides, and xenobiotics metabolism control the oscillation of the peripheral clock [50]. The metabolites act as the cellular transducer in a peripheral organ like the liver [50, 54]. The rhythmic expressions of different genes in hepatic tissue related to metabolic functions are driven by rhythmic food intake in mice [55, 56]. The transcriptional activity of many metabolic genes lost its rhythmicity during arrhythmic feeding, although core clock genes show rhythmic oscillation in the same condition. Arrhythmic feeding impairs the expression of several genes of metabolic enzymes related to carbohydrate and lipid metabolism (glycogen synthesis, cholesterol biosynthesis, and fatty acid chain elongation), as well as the expression of glucose transporter-2 (GLUT2) and GLUT4 in hepatic and adipose tissue respectively. The GLUT2 increases the intracellular glucose level in hepatocytes that is used for lipogenesis instead of glycogen synthesis [56]. They also reported that signaling pathways like mechanistic target of rapamycin (mTOR) and extracellular signal-regulated kinase 1/2 (ERK1/2) regulate the activity of metabolic transcription factors. The phasic activation of these proteins does not occur in arrhythmic feeding conditions, while the level of these proteins remains constant in hepatocytes during different feeding paradigms. Thus, mTOR and ERK1 and ERK2 mediated metabolic gene expression does not sustain in an arrhythmic feeding state. Moreover, it had been reported that CREB, forkhead box protein O1 (FoxO1), sterol regulatory element-binding protein (SREBP), and activating transcription factor 6 (ATF6) also regulate the transcription of several genes during the feeding rhythmicity. The expressions of CREB and FoxO1 mediated genes were repressed after feeding, while the reverse effect was observed in ATF6 and SREBP regulated genes [55]. Non-SCN tissues bear the local oscillatory pacemaker and the importance of the peripheral clock was studied by Kornmann et al. [57]. They observed that genetic manipulation in circadian dysfunctioning liver expresses genes related to the circadian rhythm, which are partly regulated by the liver clock and the signals from SCN.
The disruption of circadian clock function was associated with several diseases; these include sleep disorders, metabolic dysfunction, infertility, cancer, and psychological disorders. Modern lifestyle, excessive workload, depression, shift work, exposure to excessive artificial light, and short night periods severely affect the circadian clock functions and promote the initiation of circadian mediated health disorders. According to the World Health Organization, shift workers are very prone to health risks [58].
Human circadian rhythm disorders are related to the activity of the internal clocks and the external LD cycle. The American Academy of Sleep Medicine has classified circadian rhythm sleep disorders (CRSDs) into several categories. These include advanced sleep phase disorder/syndrome (ASPD/ASPS), delayed sleep phase disorder/syndrome (DSPD/DSPS), free-running disorder (FRD), and irregular sleep-wake rhythm (ISWR) [26]. The features of these disorders have given in Table 1. Sleep disorders are also common phenomena in jet lag and shift work, but they are treated as extrinsic disorders and do not relate to circadian clock dysfunction. The jet lag and shift work-mediated CRSDs occur due to voluntary or imposed shifts in the sleeping time. Several non-communicable disorders like stroke, mania, depression, intracranial infection, head injury, and central nervous system (CNS) stimulant or depressant drugs are associated with CRSDs [26].
Four common sleep disorders and their features [26]
| Name | Features | Prevalence |
|---|---|---|
| ASPD/ASPS, ~2% | The sleep episode occurs in advance time than the desired clock time, leading to early sleep onset and early awakening than the desired time | ASPD is more prevalent in elderly people |
| This syndrome is rare and has a genetic basis. It is conferred by a single autosomal dominant gene that resides on the short arm of chromosome 2 which carries Per2 genes. Mutation results from serine-to-glycine in PER2 protein are the responsible party for this syndrome | ||
| Mutation in CK1ε lowers the rate of degradation of PER2 protein. Misregulation of PER2 protein promotes ASPS | ||
| DSPD/DSPS, 83% | The onset of sleep and final awakening are delayed concerning the desired clock time. The body temperature, melatonin, and sleep rhythm of DSPS subjects are very similar to normal subjects, but the phase is delayed. They are less productive in their profession and social life | DSPS may appear in early childhood but commonly occurs during adolescence and in middle age people. Social and occupational impairment can promote DSPS |
| FRD, ~2% | It is known as a non-24-h-sleep-wake syndrome and appears as a chronic disorder. In this pattern, progressive changes of 1-h to 2-h delays in sleep onset and wake times are the fundamental features | FRD is common in those who are blind and it may be due to failure of the entrainment to the LD cycle |
| The variable period length indicates the interaction between the environment and the sleep-wake cycle | ||
| ISWR, 12% | The pattern is temporally disorganized. The variable episodes of sleeping and waking behavior are very common. A short sleep period (a few hours) is distributed randomly throughout the day and night | ISWR is very common in night workers, shift workers, students of colleges, universities, technical institutes, medical schools, law schools, and research institutes |
| It has also been observed mainly in the mentally retarded and demented individuals |
Neurodegenerative diseases are associated with progressive loss of vulnerable neurons in the brain, leading to decreased brain functions. Progressive loss of memory and cognitive impairments, and difficulty in moving and speaking are the common symptoms of neurodegenerative diseases [59, 60]. Neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), Pick’s disease, and progressive supranuclear palsy (PSP) are bidirectionally associated with circadian disruption [61–67]. Accumulation of insoluble misfolded or mutant protein aggregates like α-synuclein in PD, β-amyloid plaque and tau in AD, and huntingtin in HD, occur in these diseases [68]. Proteotoxic stress, impaired activity of ubiquitin–proteasomal and autophagosomal/lysosomal systems, mitochondrial dysfunction, glutamate toxicity, neuroinflammation, aging, and circadian dysfunction accelerate neurodegenerative disorders [63, 66, 69–71].
The circadian clock regulates the rhythmic expression of numerous genes and is also involved in protein processing and transmission of protein aggregates [68, 72, 73]. Circadian dysfunction promotes the formation of misfolded-protein aggregates and their transport. A proper sleep pattern can help to remove the misfolded protein. However, circadian deregulation also causes sleep disorders that accelerate the pathology of neurodegenerative diseases. At the experimental level, a study on postmortem tissue of patients with neurodegenerative disorders shows disruption in rhythmic expression of circadian genes [74]. This finding is also similar to the aging brain [75].
Disruption of circadian clock functions enhances oxidative stress (OS) that induces neurodegeneration [76]. In the brain, BMAL1 regulates the transcription of redox-related genes like nicotinamide adenine dinucleotide phosphate (NADPH) dependent quinone dehydrogenase (QR2), and aldehyde dehydrogenase 2 (ALDH2). The expression of these genes essentially regulates OS and neuronal damage [77, 78]. The clock components also interact with silent information regulator transcript 1 [SIRT1, nicotinamide adenine dinucleotide (NAD)-dependent deacetylase sirtuin 1] for maintaining the cellular redox homeostasis [79] and the transcription of both CLOCK and BMAL1 in SCN [80]. Dysfunction of circadian rhythm and antioxidant activity influences PD and AD [66, 81].
Circadian dysfunction also decreases melatonin secretion. Melatonin is considered the hormone of darkness and shows its peak at midnight. This indolamine is involved in several regulatory functions and acts as a potent endogenous antioxidant. Melatonin and its derivatives reduce OS and protect the cells against OS-induced damage, maintain the proliferation of normal cells and prevent apoptosis-mediated cell degeneration [82, 83]. Shift work and night work hamper melatonin secretion and decrease its protective role against stress-induced various diseases, including neurodegenerative disorders. Moreover, circadian dysfunction alters the pattern of rhythmic secretion of different hormones from the pituitary. The output signal from the SCN regulates the activity of the hypothalamic-pituitary axis and secretion of tropic hormones. Misalignment of circadian rhythm increases the secretion of adrenocorticotropic hormone (ACTH), followed by the release of cortisol. Excess cortisol disrupts the sleep-wake cycle that promotes neurodegeneration [84].
Formation of neurofibrillary tangles and deposition of extracellular amyloid beta (Aβ) have been observed in AD patients. Missense mutations, single nucleotide polymorphisms (SNPs), and deregulated alternative splicing promote malformed tau protein synthesis [85, 86], which is very unstable and fails to bind with microtubule [87]. This protein accumulates as phosphorylated tau that contributes to neurofibrillary tangles formation. β-secretase and γ-secretase cleave the amyloid precursor protein to generate Aβ which forms β-amyloid plaques [88, 89]. Ma et al. [90] reported that circadian dysfunction increased Aβ synthesis by increasing β-secretase activity. Wang et al. [91] reported that Aβ altered Per1 and Per2 expression in SCN, which are associated with AD. Experiments on drosophila indicated that sufficient expression of PER exerted neuroprotection against oxidative damage [92]. PER also maintains the sleep-wake cycle and working memory [93]. Ablation of Per decreases antioxidant activity and increases OS that collectively influences neurodegeneration [92, 94]. Altered light exposure-mediated circadian desynchrony promotes AD in mice due to alteration in the expression of SIRT1 and activation of OS pathways in the hippocampus [95]. Aβ promotes the degradation of BMAL1, followed by circadian deregulation and advancement of AD [96]. Kress et al. [97] reported that Bmal1 knockout increases Aβ plaque formation. Deregulated expression of BMAL1 also disrupts PER and CRY expression and redox homeostasis that contributes to AD [98]. Li et al. [99] reported that CK1δ promotes phosphorylated tau deposition and neurofibrillary tangle formation. Administration of CK1 inhibitor protects the prefrontal cortex and hippocampus from Aβ plaque formation [100]. Thus, CK is also associated with AD development.
Circadian dysfunction and neuroinflammation are the common risk factor for PD. PD patients show deposition of α-synuclein as Lewy bodies. It has been suggested that α-synuclein is involved in the transport of synaptic vesicles [101]. However, aggregation of misfolded α-synuclein causes PD. A study on C57BL/6 mice in abnormal LD cycles (20:4 LD) exhibits neuroinflammation and poor motor skills. Kudo et al. [102] reported that circadian dysfunction overexpresses α-synuclein in a mouse model of PD. SNPs study in PD patients indicated a greater number of SNPs in Bmal1 and Per1 gene [103]. Although circadian dysfunction and neurodegenerative diseases are bidirectionally associated, the mutation in the Parkin gene (PRKN) increases the expression of CLOCK, CRY1, and CRY2 and decreases PER2 output. Additionally, mutant PRKN decreases mitochondrial oxygen consumption and energy production [104]. Breen et al. [105] observed the arrhythmic expression of BMAL1, PER2, and Rev-Erb α occurs in the sample taken from PD patients. Liu et al. [106] reported that deregulation of BMAL1 activity occurred in 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-treated mice, resulting in the reduction of dopaminergic neurons, decreased activity of tyrosine hydroxylase, and low levels of dopamine. Thus, improper BMAL1 activity may potentiate the pathogenesis of PD. The formation of mutant huntingtin (mHTT) protein initiates the pathologies of HD. Deregulated PER1 and PER2 expression had been observed in mice with HD [107, 108]. Smarr et al. [109] observed that SCN neurons decreased daytime firing in HD models. PSP patients exhibit poor sleep quality, sleep apnea, restless leg syndrome, and difficulty in falling asleep and staying asleep [110]. Circadian dysfunction influences PSP. CK1δ is also involved in neurofibrillary tangle formation in PSP and Pick disease [111].
Circadian disruption is associated with shift work, night work, jet lag, and exposure to outdoor light at night. A significant night-time light pollution occurs due to artificial light, which is a serious problem in different metro cities throughout the world. Light-at-night desynchronizes the circadian rhythm that promotes psycho-behavioral disorders along with several negative consequences for health. Seasonal affective disorder (SAD) may occur due to alteration of the LD cycle in the natural system during seasonal variation. Short day lengths in winter and prolonged day-light during long summer days have been observed in boreal and austral countries. People of this region can suffer from dysthymia during the short day lengths and euthymia during the long summer days.
Circadian disruption hampers the regular sleep-wake cycle and also suppresses melatonin secretion and night-time melatonin peak. The nocturnal melatonin peak maintains different physiological activities, including sleep cycle, hormone secretion, neurological activity, and psychological behavior. Impaired melatonin secretion advances different psychological disorders such as SAD, bipolar disorder (BD), unipolar depression, schizophrenia, autism spectrum disorder (ASD), and attention deficit hyperactivity disorder (ADHD) [112–114]. Treatment with melatonin in pharmacological doses improves psychiatric problems [115, 116]. Circadian rhythm dysfunction deregulates the sleep-wake cycle that influences psychological disorders. ASD is very common in children and is associated with a desynchronized sleep-wake cycle. Lai et al. [117] also reported that sleep disorders are a common problem in patients with autism. Daytime sleepiness in children increases the risk of ADHD [118].
Several authors discussed the relationship between circadian rhythm dysfunction and psychiatric disorders, specifically depression and mood problems [119–123]. Jet lag is a common problem for international flight crews. Sack [124] suggested that mood changes, particularly dysphoric mood, are associated with jet lag. Experimental data indicated that an eastward journey over a month promoted sleep deprivation and increased the risk of anxiety and depression [125]. Major depressive disorder (MDD) is a disease of alteration in mood and is associated with increased sadness and irritability. Alteration in sleep, sexual desire, or appetite, inability to experience pleasure, slowing of speech or actions, crying, and suicidal thoughts are the symptomatic changes in the patients [126]. Several reports suggested that shift work and night work-dependent circadian rhythm dysfunction increases the outcome of MDD. Jet lag or social jet lag also influences MDD [122]. Decreased latency to rapid eye movement (REM) sleep, duration of slow-wave sleep and increased duration of REM sleep, disruption of melatonin and cortisol rhythms, and elevated nocturnal body temperature are associated with MDD [127]. Studies with postmortem brains of patients with MDD revealed that the core clock genes had expressed in reduced amplitude, shifted peaks, and phases [128]. Additionally, bilaterally ablation of SCN decreases immobility during forced swim tests on rats [129]. Applications of bright light therapy, wake therapy, social rhythm therapy, and antidepressants can improve MDD [122]. SCN-specific Bmal1-knockdown in mice and exposure to T22 LD cycle-induced desynchrony of the SCN in rats accelerates depressive-like behavior [130, 131].
Circadian rhythm disruption can increase the risk of anxiety. A study reported that non-shift workers had started to suffer from anxiety if they were facing rotating shift work schedules [132]. Similarly, nurses also showed higher anxiety scores on the Hospital Anxiety and Depression Scale [133]. Improper expression of core clock components influences anxiety-like disorders. Delta19 (Δ19) mutations in the Clock gene of mice decreased the symptoms of anxiety [134]. Per1 and Per2 deficient mice showed greater anxiety-related problems; however, lack of individual expression of either Per1 or Per2 had no effects on anxiety [135].
BD is characterized by cyclical changes in mood between mania and depression separated by an interval of normal affect. The episodes of mood in BD depend on the behavior of the person, sleep cycle, physical activity, and energy expenditure. A meta-analysis study has established the relationship between circadian rhythm disruption and BD [136]. Jet lag can influence the BD. Traveling in different time zones, particularly east to west occurs depression, whereas traveling from west to east develops mania [137]. Le-Niculescu et al. [138] reported that core clock genes were associated with BD. Polymorphisms in circadian clock-associated genes increase the risk of development of BD [139]. Lithium slows down the fast-running circadian clock and normalizes the circadian rhythm, which is an effective treatment for BD. Moreover, bright light therapy can improve BD [122, 139].
Schizophrenia is a typical mental disorder. The symptoms are delusion, hallucination, thought disorders, movement disorders, anhedonia, alogia, cognitive failure, and attention deficits. There are several reasons for schizophrenia. These include genetic factors (familiar), environmental (poverty, stress, exposure to viruses, and nutritional problems before birth), imbalances in neurotransmitters, and abnormalities in brain functions. Moreover, circadian rhythm disruption and sleep disorders are also associated with schizophrenia [122, 140, 141]. Studies on postmortem brain tissues of schizophrenia revealed the arrhythmic expression of many genes in the prefrontal cortex and rhythmic expression of mitochondrial genes, compared to healthy individuals [142]. Schizophrenia individuals show hyperactivity of the hypothalamic-pituitary-adrenal axis that stimulates stress-induced activities and motor functions [143, 144]. Circadian rhythm dysfunction alters the melatonin secretion in individuals, which promotes schizophrenia [145]. SNPs studies indicated that mutation in Ror β, Per2, Per3, and neuronal PAS domain protein 2 (Npas2) were associated with schizophrenia [146]. Peripheral tissues of schizophrenia tissues also showed arrhythmic expression of Clock, Per2, and Cry1 genes [147].
Disruption of circadian rhythm is associated with cardiovascular diseases (CVD). Diurnal rhythms have been observed in heart rate, blood pressure, blood catecholamine secretion, cortisol secretion, platelet aggregation, and others. Changes in circadian rhythm modulate the sympathetic activity, secretion of catecholamines, and aldosterone biosynthesis, as the sympathetic activity controls the adrenal medullary functions as well as the renin-angiotensin system. The synergistic effect is impaired heart rate and blood pressure. Alteration in the circadian clock system also changes the fuel metabolism in cardiomyocytes. Normally, nicotinamide phosphoribosyltransferase (NAMPT) regulates NAD biosynthesis. Dysregulation of NAD synthesis alters the utilization of fatty acids, the primary fuel source. This activity is mediated by the peroxisome proliferators activated receptor γ (PPARγ) signaling system. Circadian dysfunction in endothelial cells alters the activity of Akt and nitric oxide (NO) signaling. NO is essential for vascular relaxation. Dysregulation of NO activity initiates hypertension (Figure 2) [148–150].
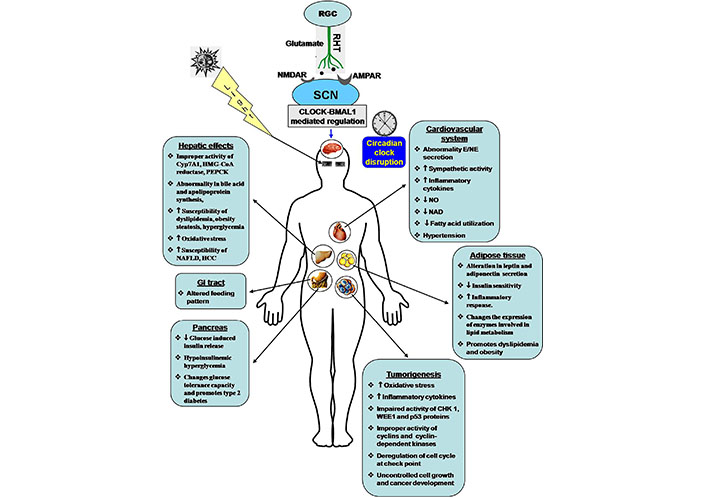
Effects of circadian clock dysfunction on different tissues and progression of various disorders. NMDAR: NMDA receptor; AMPAR: AMPA receptor; Cyp7A1: cholesterol 7α-hydroxylase; HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A; PEPCK: phosphoenolpyruvate carboxykinase; NAFLD: non-alcoholic fatty liver disease; HCC: hepatocellular carcinoma; GI: gastrointestinal; E: epinephrine; NE: norepinephrine; CHK 1: checkpoint kinase 1; WEE1: a specific G2 check point kinase; p53: tumor suppressor protein 53; ↑: increase; ↓: decrease
In the recent era, metabolic disorders are a serious problem in our society. These include obesity, hyperglycemia, type 2 diabetes, dyslipidemia, and hypertension [149, 151, 152]. Metabolic disorders alter blood triglyceride, high-density lipoprotein, and fasting blood glucose levels, as well as inflammatory markers [C-reactive protein, tumor necrosis factor-α (TNF-α), interleukin-6, and plasminogen activator inhibitor type 1] and anti-inflammatory components [149]. Circadian clock disorder is a vital contributor to cancer, diabetes, obesity, and cardiometabolic disorders (Figure 2) [153]. Various nuclear hormone receptors like PPARα, PPARγ, Rev-Erb α, Ror α, hepatocyte nuclear factor 4α (HNF4α), thyroid hormone receptor α (TRα), and nuclear receptor related 1 (NURR1) directly regulate the metabolic pathway. The circadian clock has a link with these nuclear hormone receptors. Imbalance in the circadian clock affects PPARγ-mediated lipogenesis and lipid storage, PPARα-dependent ketogenesis, and hepatic fatty acid oxidation [154]. The occurrence of type 2 diabetes mellitus and obesity are much higher in night shift workers than the day shift workers [153, 155]. An animal model study in an alternative LD cycle revealed that the profile of blood glucose, insulin, and triglyceride levels differ according to the alternative circadian system [156]. Polymorphic variations indicate that clock genes like Clock, Per2, Per3, Bmal2, Cry1, and Cry2 are responsible for metabolic disorders [157]. Mutant animals exhibit chronodisruption and disorder in lipid metabolism by changing the pattern of cholesterol metabolism, expression of lipase enzyme, and production of glycerol and free fatty acids during the circadian period; all of which are associated with adipocyte hypertrophy and dyslipidemia [158]. Gabriel and Zierath [159] reported that skeletal muscle has a strong circadian profile. Shift workers are prone to insulin resistance and type 2 diabetes. Timed exercise is most beneficial for improving health and metabolic diseases. Different organs are directly or indirectly affected by metabolic disorders (Figure 2).
The liver maintains the metabolic homeostasis in the body. The hepatic clock genes modulate several metabolic activities like bile acid synthesis, apolipoprotein synthesis, and gluconeogenesis. Clock component Rev-Erb α represses the expression of small heterodimer partner (SHP) and E4 promoter binding protein 4 (E4BP4). These two proteins suppress the expression of bile acid synthesizing enzyme Cyp7A1, which regulates cholesterol and bile acid homeostasis. Circadian clock disruption has an impact on the activity of hepatic Cyp7A1, HMG-CoA reductase, PEPCK. Thus, circadian dysfunction- mediated enzymatic deregulation increases the susceptibility to dyslipidemia, steatosis, and hyperglycemia (Figure 2) [149, 160, 161]. The reduced form of redox cofactors NAD(H) and NADP(H) have an impact on the activity of CLOCK/BMAL1 and NPAS2/BMAL1 [162]. The OS-induced reduction of NADPH levels alters the CLOCK/BMAL1-induced transcriptional activity. Circadian rhythm oscillates NAD levels in the liver. The expression of the Nampt gene is upregulated by the CLOCK/BMAL1 complex. The RNA levels of NAMPT and concentration of NAD decrease in the liver of ClockΔ19/Δ19 and Bmal1−/− mice; alternatively, CRY1 and CRY2 mutation in mice show a higher level of these two factors [149]. A high-fat diet (HFD) can alter the expression of core clock genes such as Clock, Bmal1, Per1, Per2, Cry1, and Cry2 in the SCN, hypothalamus, adipose tissue, and liver. A HFD modulates the activity of acetylated H3K27, Pol II binding, nuclear factor kappa B (NF-κB)-mediated transcriptional activity, PPARγ inducing functions, and metabolic activity of gut microbiota. The collective effects are metabolic disorder, inflammatory response, progression of type 2 diabetes, and CVD [152, 163]. Jacobi et al. [164] reported that BMAL1 regulates mitochondrial biogenesis, as well as mitochondrial fission and mitophagy in hepatic cells of mice. This activity correlates between the circadian clock and metabolic rhythm. Inactivation of the BMAL1 function hampers metabolic activities, starts mitochondrial swelling, and increases OS.
Adipose tissue influences adipogenesis in the subcutaneous visceral area. Clock genes regulate the activation of SIRT1, which increases insulin sensitivity and reduces the inflammatory response in adipocytes [149, 165]. Circadian dysregulation changes the expression patterns of enzymes involved in lipid metabolism, insulin sensitivity, obesity, as well as inflammatory response (Figure 2). Moreover, a HFD alters the functions of clock control genes and fuel metabolism. These effects disturb fatty acid mobility and contribute to lipotoxicity [149, 166]. The obesity-induced inflammatory response also initiates steatosis, NAFLD, HCC, and CVD. HFD has a great impact on adipogenesis, food consumption, and obesity. HFD and impaired expression of clock genes in adipose tissue change the pattern of fuel metabolism [166]. Deletion of Bmal1 in mouse adipocytes modulates the adipose functions and the activity of lateral hypothalamic neurons of the feeding center to increase food consumption [167]. Previously, Xu et al. [168] reported that disruption of clock genes in fat cells of Drosophila increases starvation sensation and food consumption. Another report indicated that clock genes knockout mice have a prevalence of obesity [153].
Normally, leptin regulates feeding rhythm. Rhythmic expression of leptin has been observed in mice, which oscillates along with the LD cycle [169]. Alternatively, 24 h leptin and adiponectin profiles do not maintain in obese humans [170]. Leptin and adiponectin exert their signaling via PKA in muscle and liver [171]. Adipose tissue secretes adipokines, which influence proinflammatory activity. Obesity induces the secretion of interleukin-6, TNF-α and also increases the activity of the endoplasmic reticulum (ER) in the liver, adipocytes, and pancreas. ER stress starts over-secretion of adipokine from adipose tissue and enhances the accumulation of reactive oxygen species [172]. Thus, impaired circadian clock function alters adipose tissue functions, particularly leptin, adiponectin, proinflammatory cytokine secretion, and OS response. The synergistic effect of these factors promotes metabolic disorders. In humans, the secretion of adipokines occurs in a circadian fashion, which is involved in the regulation of metabolic homeostasis [149, 173]. Kettner et al. [174] reported that the study of chronic jet lag in the experimental animals showed impaired leptin activity and obesity, which indicates the relationship between circadian misalignment and metabolic disorder.
Blood glucose level and insulin action rhythmically change across the day. The central clock and in association with peripheral clocks control the feeding pattern, energy output, and insulin sensitivity in the body [175]. Misalignment in the circadian clock changes the glucose tolerance capacity and promotes type 2 diabetes. The clock gene mutant mice showed hypoinsulinemic hyperglycemia (Figure 2) [153, 176, 177]. Elevated fasting glucose was observed in polymorphic CRY2 [178, 179]. Melatonin has a role in insulin secretion and inhibits glucose-induced insulin release. Impaired circadian rhythm changes the pattern of melatonin secretion and reduces the glucose-induced insulin release from pancreatic β-cells, leading to hyperglycemia [180]. CLOCK-BMAL1 complex interacts with the circadian rhythm-regulating distal regions in pancreatic β-cells and directly influences insulin synthesis and release [181].
CLOCK-BMAL1 complex also regulates the expression of NAMPT and SIRT1. Both are essential for the biosynthesis of NAD, gluconeogenesis in the liver, glucose-induced insulin secretion from the pancreas, and glucose metabolism [149]. Impaired glucose tolerance had observed in Nampt-deficient (Nampt−/−) mice. Intra-peritoneal injection of nicotinamide mononucleotide (NMN) improves the condition [182]. However, overexpression of SIRT1 in pancreatic β-cells exhibited better glucose tolerance and insulin secretion [183]. Stenvers et al. [175] reported that chronodisruption was a factor in the development of insulin resistance, followed by the progression of type 2 diabetes. Core clock gene mutation, shift work or night work, exposure to artificial light-at-night, and sleep disturbance impact glucose metabolism and insulin sensitivity. Thus, dysfunction of the biological clock decreases insulin release, insulin activity, and glucose metabolism, leading to the advancement of the conditions of type 2 diabetes.
SCN-mediated dysfunction of circadian events advances cancers in the different tissues. Clock components regulate the expression of factors related to the cell cycle, DNA repair, metabolism, redox homeostasis, and others. They control the secretion of cytokines, hormones, neurotransmitters, tumor-specific metabolites, and the activity of oncogenic proteins. Collectively, disruption of circadian function alters cellular functions, leading to the progression of cancer development (Figure 2) [184]. In our modern society, people are involved in shift work and night work. They have been exposed to bright light-at-night in industrial sectors, the medical profession, transport services, and others, resulting in misalignment of circadian rhythm that leads to disruption of the sleep-wake cycle, metabolic disorder, and cancer progression. The epidemiological and experimental studies revealed that circadian dysfunction was associated with cancer. International Agency for Research on Cancer (IARC) has categorized the shift-work as “carcinogenic to humans (Group 2A)” [185]. A recent report indicates that night shift work enhances the risks of breast, prostate, and colorectal cancer [186–188].
The study of SNP indicates the role of clock genes in disease progression, including cancer [6, 28, 157, 189]. Any alteration or improper expression of clock genes such as Clock, Bmal1, Ck1ε, Cry1–2, Npas2, and Per1–3 promotes the formation of different cancers like breast, colorectal, prostate, pancreatic, and non-Hodgkin lymphoma [6, 28, 190]. Deregulation of expression of the core clock genes Per1, Per2, Per3, Cry1, Cry2, Bmal1, and Clock are associated with the development of cancer in humans [30, 191]. Impaired expression of Per1, Per2 and/or Per3 had been observed in several cancers, including breast [191, 192], prostate [193, 194], oral [195], pancreatic [196, 197], hepatocellular [198], colorectal [199], non-small cell lung cancer [200], renal [201], bladder [202], myeloid leukemia [203, 204], glioma [205], and squamous cell carcinoma [206]. The cell cycle suppressor protein WEE1 is also regulated by PER and CRY. Alteration in Cry expression deregulates the synthesis of WEE1 (a specific G2 check point kinase, cell cycle suppressor protein) and cyclin D1; the result is impaired cell proliferation and tumor growth [207, 208]. Mutation in the Cry2 gene increases the risk of lymphoma [209]. Valenzuela et al. [157] reported that polymorphisms of Clock, Per2, Per3, Bmal1, Cry1, Cry2, Tim, and Ror β genes are associated with breast colorectal and prostate cancer. Variants of Cry genes increase the risk of non-Hodgkin’s lymphoma [209, 210]. Circadian disruption causes dyslipidemia as well as NAFLD [211]. SNP of Per3 and Cry1 genes promotes HCC [212]. Shift work changes the lifestyle and pattern of metabolism that cause NAFLD and then non-alcoholic steatohepatitis (NASH); the result is hepatic fibrosis followed by cirrhosis and finally HCC [213]. Bile acid can influence the progression of HCC [211]. Circadian disruption affects cholesterol, bile acid, and xenobiotic metabolism. Bile acids promote non-genotoxic HCC by inducing NAFLD, and NASH. Chronodisruption increases the risk of ovarian cancers in females who are involved in night shift work. This might be associated with inappropriate expression of circadian genes [214]. Jim et al. [215] reported that variation in Arntl, Cry2, CK1ε, NpasS2, Per3, and Rev1 genes influences ovarian cancer.
Human infertility disorder is multifactorial, and one-third of the cases are male-associated. The activity of the reproductive system depends on the expression of the steroidogenic acute regulatory protein (STAR), rate of steroidogenesis, and luteinizing hormone (LH) surge before ovulation in females; while maintenance of sperm attributes in males are also crucial. All these activities are directly or indirectly associated with clock gene expression, as the SNP of Clock and Bmal1 is correlated with infertility [216–218].
In our modern life, several activities such as medical services, uninterrupted power supply, operation of nuclear power plants, transport services, monitoring of satellite movements and the space shuttle, continuous operation of industrial work, and military activities are going on round the clock. In these sectors, people are working on different shifts. Sometimes the shifts are extended due to a shortage of manpower, and demand for service. The shifting duties, as well as extended shift, disrupts the circadian clock functions and various physiological rhythms (sleep cycle, melatonin secretion, core body temperature regulation, ACTH/cortisol secretion, plasma triacylglycerol, glucose homeostasis, and metabolic activities) of the body [14, 35]. Disruption of circadian rhythm is detrimental to health, particularly for CVD [219]. Circadian dysfunction also decreases alertness, cognitive functions, mood, social activities, and performance of physical work of an individual [220, 221]. Physical performance of the body depends on hormone secretion, core body temperature, and metabolic activity. The healthy individuals with normal LD cycles (12:12 LD) exhibit the highest physical performance and work efficiency in the daytime. The work capacity tends to decrease from the evening and reaches the minimum level within the period 2.00 A.M. to 4.00 A.M. when the core body temperature remains at the lowest value [222]. During this period, the body feels fatigued, which increases the chance of an accident.
The continuous phase changes in shift work and night work (about 20% in the industrialized nations) cause potential health hazards, and safety problems [219]. Many accidents (road, industrial, power plant) happen due to ill adaptation of circadian clock functions, feeling of fatigue, poor physical performance, decreased alertness, and mental disturbance [223–225]. A survey-based study related to the rate of vehicle accidents revealed that the number of accidents/collisions maximally happened after 2.00 A.M. Despite many reasons for the accident, the dysfunction of circadian events could not be ignored [226]. The major disastrous events like the Exxon Valdez supertanker accident in Alaska, the Chernobyl disaster (26th April 1986 at 1.23 A.M.), and the U.S. commercial nuclear power plant accident at 3 Mile Island, Pennsylvania (28th March 1979 at 4.00 A.M.) happened at the late night and circadian dysfunction mediated poor physical performance might be associated in these mishaps [226–228].
Jet lag is a transient disruption of the circadian system during transmeridian flights. It is the result of a temporary loss of synchrony between biological clock functions and an alternate LD cycle in the new time zone. The result is an abrupt change in the sleep-wake cycle according to the local time. Jet lag disrupts the feeding/fasting cycle, rest/activity cycle, and sleep-wake cycle [35]. The common symptoms of jet lag are fatigue and sleepiness during the day in the new time zone, inability to satisfactory sleep at night, loss of appetite, indigestion, cognitive distress, inability to co-ordinate with activity, loss of motivation, irritability, headache, and obesity [229–231]. The exogenous and endogenous components of the circadian system are mismatched until the internal clock is gradually re-entrained. The profiles of the body temperature, sleep-wake cycle, and endocrine functions have readjusted to local time, and the discomforts of jet lag disappear slowly. Recently, the use of melatonin is more popular to adjust the circadian rhythm rapidly. Light treatment may also give beneficial effects. However, light treatment and melatonin treatment counteract each other as melatonin production is reduced by light [232, 233]. Chronic jet lag is a common phenomenon for shift workers, flight attendants, and flight crews. Different studies indicated that chronic jet lag influences the elevated level of cortisol, poor cognitive functions, low working memory, learning problems, and higher rates of breast and prostate cancer [234, 235].
The circadian system acts as a biological timekeeper and regulates physiological functions throughout the day in relation to environmental time cues. The primary activity of the circadian system occurs within the SCN, which makes synchronization with the endogenous peripheral clocks. SCN receives a light-associated impulse from the retina that governs the expression of the core clock genes. Misalignment in the circadian rhythm adversely affects human health. There are many anthropogenic causes of circadian clock dysfunction; these include rotating shift work, night work, exposure to bright light-at-night, chronic jet lag, and modern lifestyle. Circadian dysfunction influences the development of sleep problems, neurodegenerative diseases, psychological illness, metabolic syndrome (diabetes, dyslipidemias, obesity, etc.), cardiovascular disorder, and cancer. In recent times, these disorders are the major public health issues and people are facing serious problems in this concern.
Proper maintenance of circadian rhythm is essential for health benefits as modern society is tremendously affected by artificial light, workload, jet jag, shift work, etc. Understanding the molecular mechanism of clock functions provides an opportunity for the development of chrono-pharmacology that can reset the biological clock to a new time zone. This approach will be helpful for the treatment of metabolic disorders, jet lag problems, sleep disorders, and some neuropsychiatric illnesses. Nocturnal lifestyles increase the chance of cancer progression. An approach to regulate circadian functions would be a preventive measure against cancer development. Managing external factors like the exposure to artificial light (timing, amount, and spectral composition), the timing of meal or feeding patterns, changing work schedules, and proper lighting at the workplace and home will be the preventive measure against the adverse effects of chronodisruption. Integrative research on epidemiological data, molecular aspects of circadian functions, and circadian dysfunction-related health problems will open new avenues for the study of several health issues and the development of the treatment strategies of chrono-medicine. Finally, it can say that increased public awareness about circadian dysfunction mediated disorder and an approach to maintain the normal alignment of the circadian clock will improve physiological functions and give health benefits in the near future.
Aβ: amyloid beta
AD: Alzheimer’s disease
ASPD: advanced sleep phase disorder
BD: bipolar disorder
BMAL1: brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1
CK1: casein kinase 1
CLOCK: circadian locomotor output cycles kaput
CREB: cyclic adenosine monophosphate response element binding protein
CREs: calcium/cyclic adenosine monophosphate response elements
CRSDs: circadian rhythm sleep disorders
Cry: cryptochrome
CVD: cardiovascular diseases
Cyp7A1: cholesterol 7α-hydroxylase
ERK1/2: extracellular signal-regulated kinase 1/2
Fbxl3: F-box/LRR repeat protein 3
FRD: free-running disorder
GLUT2: glucose transporter-2
HCC: hepatocellular carcinoma
HD: Huntington’s disease
HFD: high-fat diet
HMG-CoA: 3-hydroxy-3-methylglutaryl coenzyme A
ISWR: irregular sleep-wake rhythm
LD: light-dark
MDD: major depressive disorder
NAD: nicotinamide adenine dinucleotide
NADPH: nicotinamide adenine dinucleotide phosphate
NAFLD: non-alcoholic fatty liver disease
NAMPT: nicotinamide phosphoribosyltransferase
NO: nitric oxide
Npas2: neuronal period-aryl hydrocarbon receptor nuclear translocator single-minded protein 2
OS: oxidative stress
PD: Parkinson’s disease
PEPCK: phosphoenolpyruvate carboxykinase
Per1: Period 1
PPARγ: peroxisome proliferators activated receptor γ
PSP: progressive supranuclear palsy
Rev-Erb: reverse-erythroblastosis
RHT: retinohypothalamic tract
Ror: retinoid-related orphan receptor
RORE: retinoid-related orphan receptor response element
SCN: suprachiasmatic nucleus
SIRT1: silent information regulator transcript 1
SNPs: single nucleotide polymorphisms
The author is grateful to Midnapore College, Midnapore, West Bengal, India, for providing all kinds of facilities to prepare this manuscript. The author expresses his sincere thanks to Dr. Amal Kanti Chakraborty, Ex-Associate Professor of English, Midnapore College Midnapore, for his convenient help during the linguistic correction.
SAA initially started literature searching and preparation of the manuscript. SS guided the overall work. He involved in the writing and correction of the manuscript and gave the final shape of this review article.
The authors declare that there is no conflict of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Georges Maestroni
Utku Aykan ... Canan Uluoglu
Viktória Vereczki ... Ágnes Csáki
Saptadip Samanta, Debasis Bagchi
