Affiliation:
1Department of Neurology, Indiana University Health, Indiana University, Indianapolis, IN 46202, USA
ORCID: https://orcid.org/0009-0002-9385-9756
Affiliation:
1Department of Neurology, Indiana University Health, Indiana University, Indianapolis, IN 46202, USA
Email: miller89@iupui.edu
ORCID: https://orcid.org/0000-0002-4226-4835
Affiliation:
1Department of Neurology, Indiana University Health, Indiana University, Indianapolis, IN 46202, USA
ORCID: https://orcid.org/0009-0002-9652-197X
Affiliation:
1Department of Neurology, Indiana University Health, Indiana University, Indianapolis, IN 46202, USA
ORCID: https://orcid.org/0000-0002-1107-6823
Affiliation:
1Department of Neurology, Indiana University Health, Indiana University, Indianapolis, IN 46202, USA
ORCID: https://orcid.org/0009-0001-1811-7878
Affiliation:
1Department of Neurology, Indiana University Health, Indiana University, Indianapolis, IN 46202, USA
ORCID: https://orcid.org/0000-0003-1553-9781
Affiliation:
1Department of Neurology, Indiana University Health, Indiana University, Indianapolis, IN 46202, USA
ORCID: https://orcid.org/0000-0003-4349-674X
Affiliation:
2Department of Radiology and Imaging Sciences, Indiana University School of Medicine, Indiana University, Indianapolis, IN 46202, USA
ORCID: https://orcid.org/0000-0001-5224-1891
Affiliation:
1Department of Neurology, Indiana University Health, Indiana University, Indianapolis, IN 46202, USA
ORCID: https://orcid.org/0009-0000-5381-9181
Affiliation:
3Department of Pediatric Neurosurgery, Ann & Robert H. Lurie Children’s Hospital, Chicago, Illinois 60611, USA
4Department of Pediatric Neurosurgery, Northwestern University Feinberg School of Medicine, Chicago, Illinois 60611, USA
ORCID: https://orcid.org/0000-0002-1865-9888
Explor Neurosci. 2024;3:198–206 DOI: https://doi.org/10.37349/en.2024.00044
Received: April 03, 2024 Accepted: May 07, 2024 Published: May 31, 2024
Academic Editor: Jinwei Zhang, University of Exeter Medical School, UK
The article belongs to the special issue Epilepsy
New onset refractory status epilepticus (NORSE) is an etiologically heterogeneous condition that is associated with high morbidity and mortality. NORSE is often refractory to medical management prompting a workup for epilepsy surgery. Because NORSE remains etiologically elusive in most cases, surgical evaluations are challenging, especially when the epileptogenic zone (EZ) is not easy to lateralize as can be seen in frontal lobe seizures. Lateralizing a frontal lobe EZ may be challenging due to bilateral synchrony from commissural connections through the corpus callosum and low spatiotemporal resolution of the scalp electroencephalography (EEG). We report a pediatric patient with NORSE presenting with focal impaired awareness seizures clustering into super refractory status epilepticus (SRSE). She required surgical intervention for the treatment of her seizures after failing therapeutic doses of antiseizure medications, anesthetic drips, immunomodulation with methylprednisolone, intravenous immunoglobulin and anakinra, and the ketogenic diet. Despite her semiology being focal, the seizures were not well lateralized on scalp EEG and during phase 2 stereo-EEG (sEEG). Anterior magnetic resonance-guided laser interstitial thermal therapy corpus callosotomy (MRgLITT CC) was performed in a multistage surgical approach to successfully lateralize the EZ with a left-lateralized ictal pattern seen after reimplantation of sEEG electrodes. Our case suggests that minimally invasive MRgLITT CC can be successfully used to lateralize an EZ in frontal lobe epilepsy and that epilepsy surgery should be considered in patients with NORSE with SRSE. We also demonstrate that laser interstitial thermal therapy (LITT), while not always resulting in seizure freedom, can sufficiently disrupt a network to abort status epilepticus and lead to seizure improvements.
New onset refractory status epilepticus (NORSE) is a heterogeneous condition that results in super RSE (SRSE) requiring multiple therapeutic doses of antiseizure medications, anesthetic coma, prolonged intensive care unit (ICU) stays, immunomodulation, dietary therapies, and epilepsy surgeries [1]. Medical management alone is generally insufficient for seizure freedom [1]. NORSE often remains etiologically elusive despite extensive workup for infectious, structural, metabolic, paraneoplastic, autoimmune, and genetic causes [1]. Respiratory complications during mechanical ventilation result in 50% of deaths [1, 2]. Surgery is considered when epilepsy is drug-resistant, defined as failure of therapeutic doses of two anticonvulsant medications with unique mechanisms [2, 3]. The ideal surgical candidate has a focal epileptogenic zone (EZ) amenable to resection or stereotactic thermal ablation [4]. Lateralizing the EZ in frontal lobe epilepsy is challenging due to secondary bilateral synchrony from commissural connections through the corpus callosum and low spatiotemporal resolution of the scalp electroencephalography (EEG) [4]. Stereo-EEG (sEEG) can lateralize and localize the EZ, though bilateral homologous ictal patterns are seen [4]. Corpus callosotomy (CC) lateralized an EZ in frontal lobe epilepsy in an adult patient with atonic seizures when bilateral ictal patterns on sEEG were encountered [5]. Both CC and magnetic resonance-guided laser interstitial thermal therapy (MRgLITT) CC are therapeutic for tonic and atonic seizures with fall injuries in pediatric patients and have lateralized interictal discharges as well [6, 7]. We report a pediatric patient with NORSE presenting with focal impaired awareness seizures clustering into SRSE requiring definitive surgical intervention for treatment of her seizures following therapeutic doses of anticonvulsant medications, anesthetic drips, immunomodulation, and an adequate trial of the ketogenic diet. The seizures were not all lateralized during phase 2 sEEG. Anterior MRgLITT CC was performed in a multistage surgical approach to successfully lateralize the EZ after a bilateral ictal pattern was seen on scalp EEG and phase 2 sEEG.
A 5-year-old, right-handed girl presented with new onset status epilepticus with frequent focal impaired awareness seizures without return to baseline. She did not have a fever or intercurrent illness. Her semiology was waking up with “nightmares”, drooling with the elevation of the right arm, hyperkinetic leg scissoring, and gaze deviation to the right. Her examination was nonfocal, and she had altered mental status due to frequent seizures and did not follow commands. Her seizures were captured with prolonged EEG which showed a non-lateralizing ictal pattern with fast activity seen predominantly in the bifrontal head regions (Figure 1). She had drug-resistant epilepsy, requiring intravenous (IV) loads of levetiracetam 60 mg/kg IV, fosphenytoin 20 mgPE/kg IV, valproate sodium 40 mg/kg IV, and lacosamide 10 mg/kg IV along with trials of oral maintenance oxcarbazepine, clobazam, topiramate, and cannabidiol. Immunomodulation with a pulse dose of 30 mg/kg per day IV methylprednisolone for 5 days, an infusion of 2 gm/kg IV immunoglobulin (IVIG), and a trial of anakinra 100 mg/day subcutaneously for 5 days were unsuccessful. The ketogenic diet did not significantly improve her seizures over 1 month. She had SRSE and required anesthetic coma with burst suppression for seizure reduction utilizing midazolam, ketamine, and pentobarbital drips without complete seizure freedom. Seizures recurred frequently at 1–5 per hour with weaning of the drips. She remained in the ICU intubated and sedated. Complete blood count, complete metabolic panel, lactate, pyruvate, ammonia, and metabolic testing with free and total carnitine, acylcarnitine profile, plasma amino acids, urine amino acids, and urine organic acids were unrevealing. Lumbar puncture was normal with cell count of 3/mm3 (reference 0–5), glucose of 61 mg/dL (reference 40–70), protein of 13 mg/dL (reference 15–45), and negative culture and stain. Other infectious workups included Epstein-Barr virus, West Nile virus, St. Louis Encephalitis virus, California Encephalitis virus, Eastern Encephalitis virus, and Western Encephalitis virus titers which were unremarkable. The autoimmune and paraneoplastic encephalitis panel sent to the Mayo Clinic was negative [8]. Mitochondrial genome testing was negative, and whole exome sequencing revealed a heterozygous variant of unknown significance in the HIVEP2 gene which was determined to be non-pathogenic and shared by her asymptomatic father.
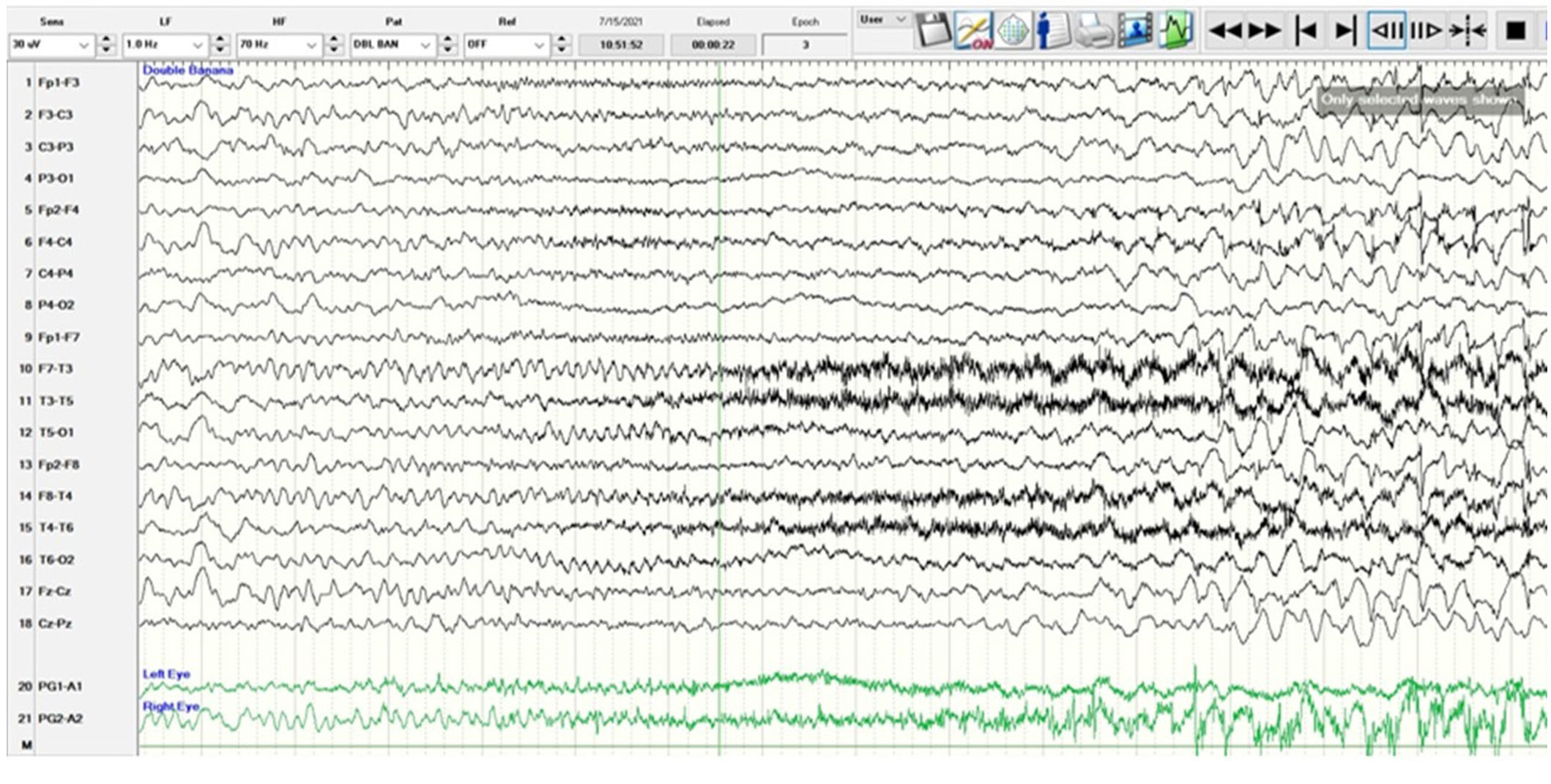
A scalp EEG in anterior-posterior bipolar montage utilizing the International 10-20 system with low amplitude rhythmic beta range frequencies which are frontally dominant starting at second 5 and best appreciated in channels 1, 5, 9, and 13 corresponding to the frontopolar head regions (Fp1, Fp2). The ictal pattern builds in amplitude, spreads, and evolves to a sharply contoured theta range activity with admixed spikes and a more organized spike-wave pattern at 3 Hz before ceasing. Semiologically, the patient raised her right arm with tonic stiffening, leg scissoring, and eye deviation to the right without a head version. The sensitivity is 30 microvolts/mm, low frequency (LF) is 1 Hz, high frequency (HF) is 70 Hz, and the epoch is 16 seconds. EEG: electroencephalography
A phase 1 workup for epilepsy surgery was performed while she was intubated and sedated in the ICU. Magnetic resonance imaging (MRI) of the brain without and with gadolinium contrast showed mild cerebral volume loss, and magnetic resonance spectroscopy was normal. Whole body positron emission tomography-computed tomography (PET/CT) was negative, and interictal PET/CT of the brain showed no hypometabolic focus, though this was suspected to be falsely negative due to frequent seizures. Ictal single photon emission CT (SPECT) revealed left hemispheric perfusion in the left occipital lobe, left parietal lobe, and left thalamus. Interictal SPECT was performed for subtracted ictal SPECT co-registered to MRI (SISCOM) which was also nondiagnostic though the Interictal SPECT was likely impacted due to frequent seizures. Following the discussion in the Comprehensive Epilepsy Program Conference at Riley Hospital for Children, she was hypothesized to have frontal lobe epilepsy, though her phase 1 workup was inconclusive for the laterality of her EZ. Due to her ictal SPECT having a wide region of hyperperfusion, sEEG was performed with a wide coverage of her bilateral frontal, temporal, and insular lobes.
A phase 2 workup in the ICU while intubated and sedated with sEEG revealed multifocal onset seizures with the left inferior frontal contacts, the left anterior insular contacts, and a bilateral ictal pattern involving the orbitofrontal contacts (Figure 2). The left inferior frontal contacts were hypothesized to be proximal to the eloquent language cortex given that she was right-handed so no ablation or resection could be performed in that region. Furthermore, cortical mapping of the left inferior frontal sEEG contacts could not be performed due to her being intubated and sedated. The left anterior insular contacts and rostrum, genu, and body of the CC were ablated utilizing MRgLITT (Figure 3). Reimplantation of sEEG revealed that the bilateral orbitofrontal ictal pattern lateralized to the left at onset following MRgLITT CC (Figure 4). Due to her seizures being focal impaired awareness seizures, MRgLITT CC did not appreciably reduce the number of seizures. MRgLITT of the left anterior insular contacts eliminated seizures from this region, confirmed by the electrocorticography measured by reimplantation of depth electrodes.
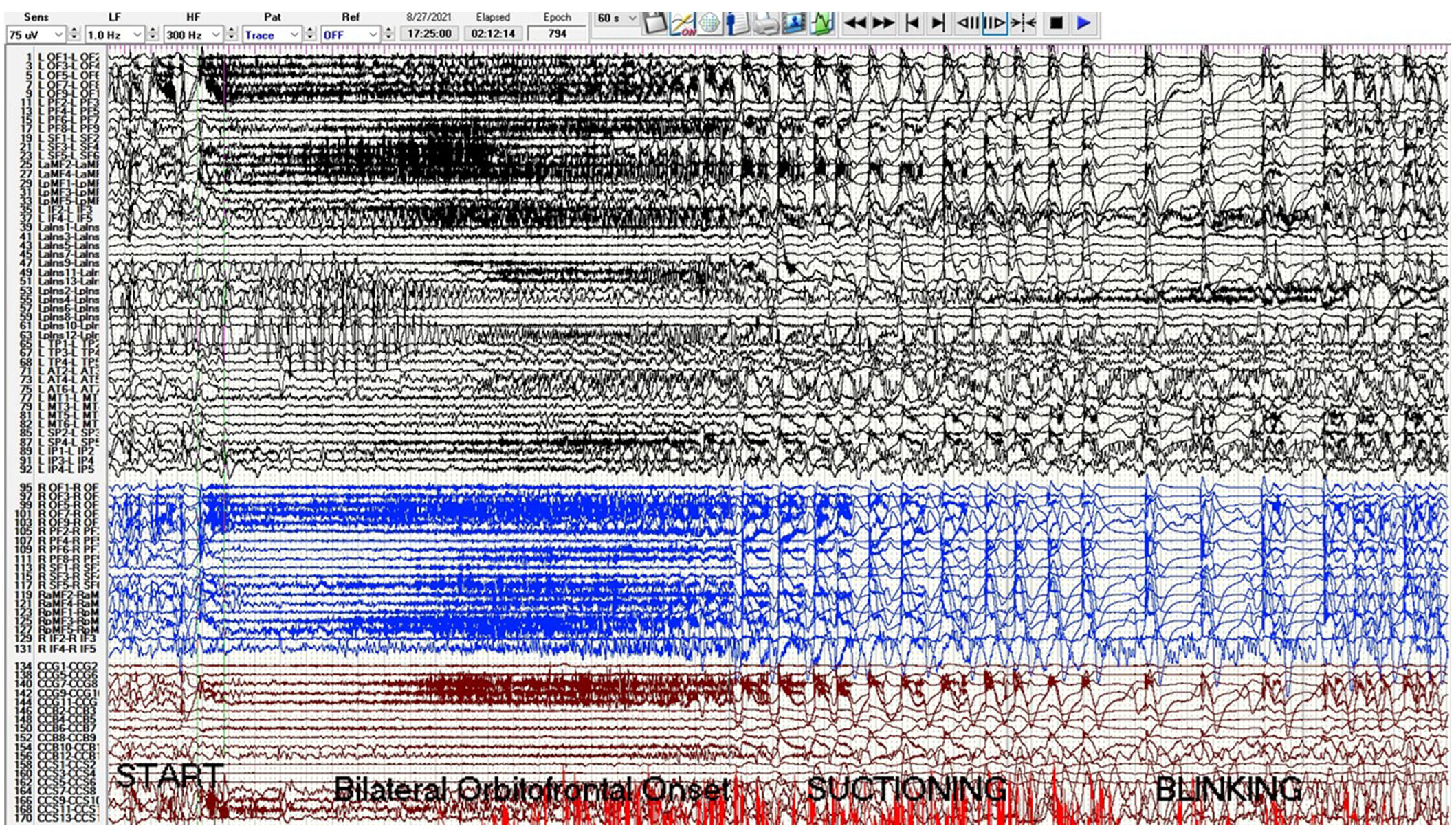
Phase 2 sEEG tracing with left channels in black, right channels in blue, and corpus callosum trajectories in red. There is a bilateral orbitofrontal and superior frontal onset seizure starting at second 20 with beta activity evolving to delta with overriding beta and then spike-wave. The sensitivity is 75 microvolts/mm, low frequency (LF) is 1 Hz, high frequency (HF) is 300 Hz, and epoch duration is 60 s. Electrode label abbreviations as follows: LOF (left orbitofrontal, 10 contacts), LPF (left prefrontal, 10 contacts), LSF (left superior frontal, 6 contacts), LaMF (left anterior middle frontal, 6 contacts), LpMF (left posterior middle frontal, 6 contacts), LIF (left inferior frontal, 6 contacts), LaIns (left anterior insula, 14 contacts), LpIns (left posterior insula, 14 contacts), LTP (left temporal pole, 6 contacts), LAT (left anterior temporal, 8 contacts), LMT (left middle temporal, 8 contacts), LSP (left superior parietal, 6 contacts), LIP (left inferior parietal, 6 contacts), ROF (right orbitofrontal, 10 contacts), RPF (right prefrontal, 10 contacts), RSF (right superior frontal, 6 contacts), RaMF (right anterior middle frontal, 6 contacts), RIF (right inferior frontal, 6 contacts), CCG (corpus callosum genu trajectory, 10 contacts), CCB (corpus callosum body trajectory, 14 contacts), CCS (corpus callosum splenium trajectory, 14 contacts). sEEG: stereo-electroencephalogram
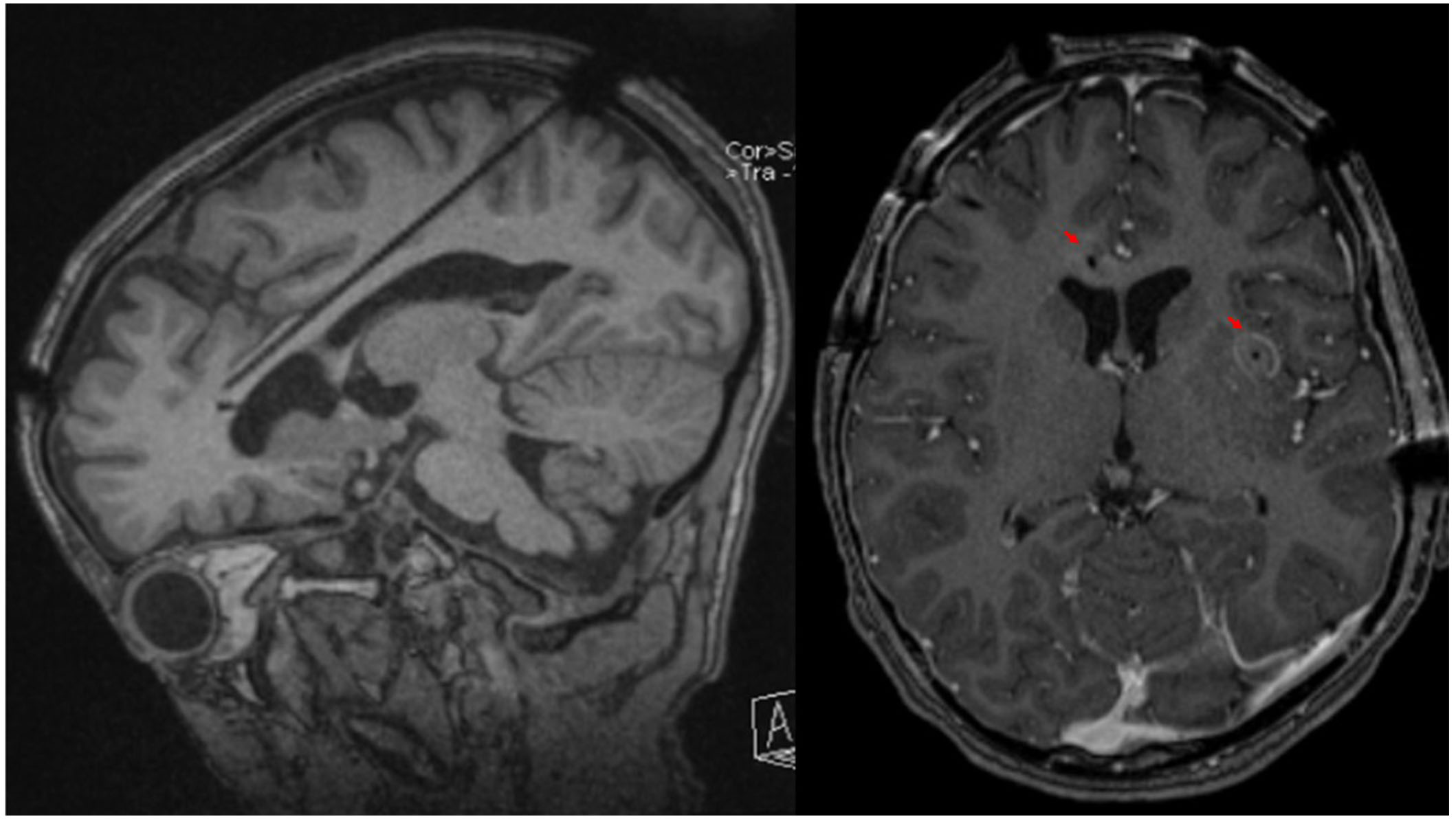
Brain MRI of probe trajectory and ablation. The left panel is an oblique sagittal T1 weighted MRI of the brain without gadolinium contrast highlighting the sagittal skew trajectory of the laser probe into the rostrum, genu, and body of the corpus callosum from a posterior approach. The right panel is an axial T1 weighted MRI of the brain with gadolinium contrast showing contrast enhancement of ablated lesions indicated by the red arrowheads in the rostrum of the corpus callosum and left anterior insula following MRgLITT CC of the rostrum, genu, and body of the corpus callosum and MRgLITT of the left anterior insula. MRI: magnetic resonance imaging; MRgLITT: magnetic resonance-guided laser interstitial thermal therapy; CC: corpus callosotomy
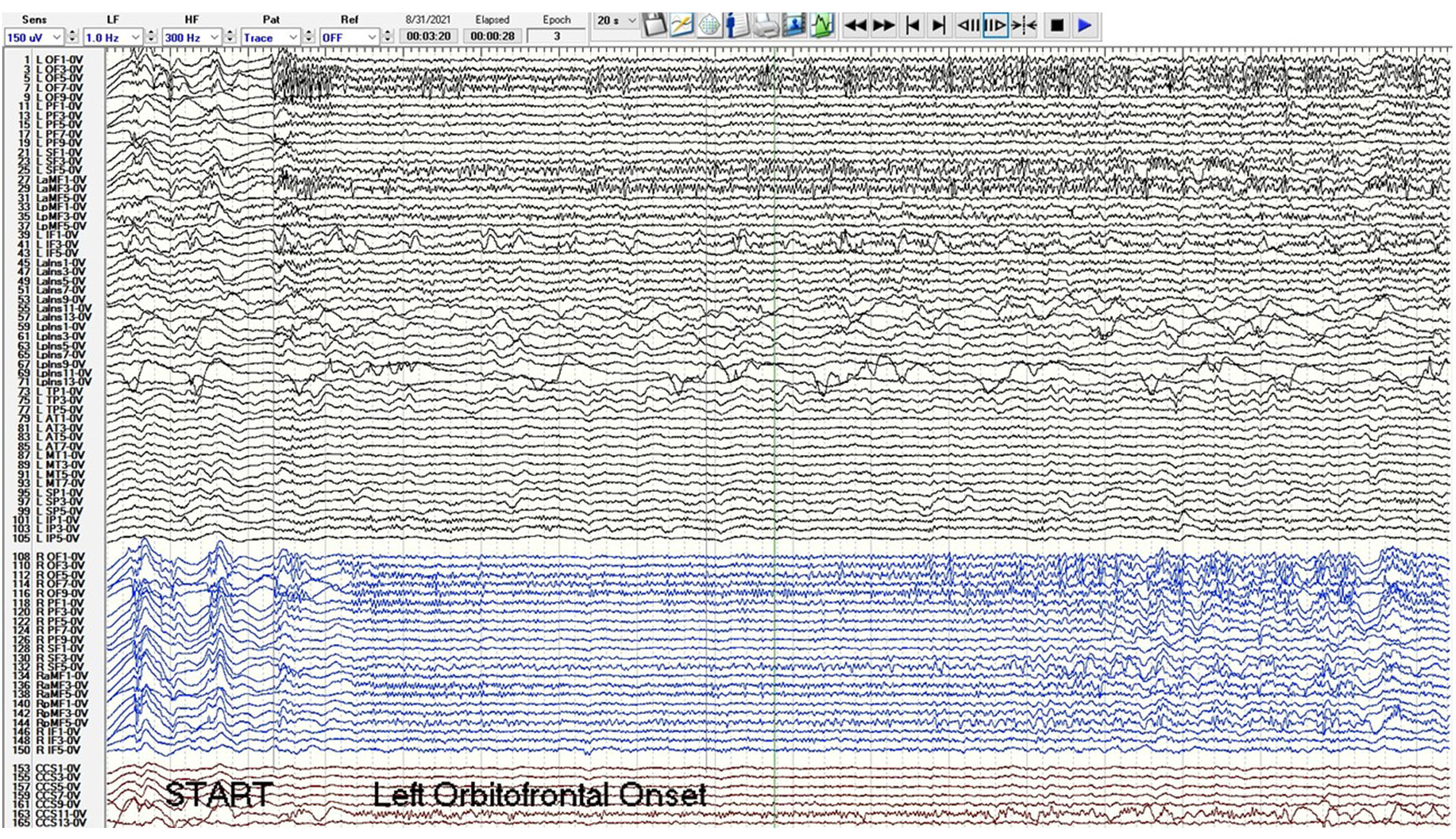
Phase 2 sEEG tracing after MRgLITT of the rostrum, body, and genu of the CC and reimplantation with left channels in black, right channels in blue, and corpus callosum trajectories in red. Post MRgLITT, the orbitofrontal and superior frontal ictal pattern lateralizes to the left orbitofrontal and superior frontal contacts at onset at second 3. The sensitivity is 150 microvolts/mm, low frequency (LF) is 1 Hz, high frequency (HF) is 300 Hz, and epoch duration is 20 s. Electrode label abbreviations as follows: LOF (left orbitofrontal, 10 contacts), LPF (left prefrontal, 10 contacts), LSF (left superior frontal, 6 contacts), LaMF (left anterior middle frontal, 6 contacts), LpMF (left posterior middle frontal, 6 contacts), LIF (left inferior frontal, 6 contacts), LaIns (left anterior insula, 14 contacts), LpIns (left posterior insula, 14 contacts), LTP (left temporal pole, 6 contacts), LAT (left anterior temporal, 8 contacts), LMT (left middle temporal, 8 contacts), LSP (left superior parietal, 6 contacts), LIP (left inferior parietal, 6 contacts), ROF (right orbitofrontal, 10 contacts), RPF (right prefrontal, 10 contacts), RSF (right superior frontal, 6 contacts), RaMF (right anterior middle frontal, 6 contacts), RIF (right inferior frontal, 6 contacts), CCG (corpus callosum genu trajectory, 10 contacts), CCB (corpus callosum body trajectory, 14 contacts), CCS (corpus callosum splenium trajectory, 14 contacts). MRgLITT: magnetic resonance-guided laser interstitial thermal therapy; CC: corpus callosotomy; sEEG: stereo-electroencephalogram
NORSE is a devastating, heterogeneous, drug-resistant epilepsy with status epilepticus refractory to conventional antiseizure medications, without structural or metabolic cause on imaging and laboratory studies, and with or without intercurrent illness or fever [1, 9]. Drug-resistant epilepsy is defined as the failure of two or more antiseizure medications for efficacy [9, 10]. Once a patient is determined to have drug-resistant epilepsy, treatments may continue with trials of additional antiseizure medications, though medical management is unlikely to result in seizure freedom even with newer-generation antiseizure medications [10]. The ketogenic diet and immunomodulation are often effective treatments in NORSE, though a broad workup should be performed to diagnose an underlying cause for guiding therapy [1, 9].
Identifying the EZ in patients with focal epilepsy is critical for definitive surgical intervention if medical, immunomodulatory, and dietary management fail [2–4]. If a lateralized EZ is not identified during phase 1 testing, patients may have staged a surgical intervention with a phase 2 evaluation and can be further studied after CC if bilateral interictal or ictal patterns are seen on sEEG [5–7, 10, 11].
The EZ is the smallest cortical region generating focal epileptic seizures [10]. Resection or ablation of the EZ leads to seizure freedom [10, 12]. An extensive network of connections afforded by the commissural fibers of the corpus callosum allows for rapid ictal spread which clinically manifests with diverse semiologies [5, 11, 13]. Bilateral homologous ictal patterns are often seen on non-invasive scalp EEG and with sEEG [5, 10, 11]. We report the case of a 5-year-old female found to have a bilateral homologous ictal pattern on sEEG which was successfully lateralized following MRgLITT CC.
Previous authors have described CC and MRgLITT CC as a palliative procedure to reduce atonic and tonic seizures, fall injuries, and epileptic encephalopathy [14–17]. Greiner et al. [14] 2012 reported a 9-year-old boy with drug-resistant epilepsy was treated with CC to prevent relapse into RSE. CC has previously lateralized interictal discharges which appeared bilateral on scalp EEG [7]. These bilateral discharges could be misinterpreted as generalized discharges, though if a lateralized EZ is suspected, discharges could be lateralized interictal discharges and determined to be focal following CC [7]. Truong et al. [5] 2014 described anterior CC successfully lateralizing the EZ in an 18-year-old female with atonic seizures and bilateral synchronous ictal activity in the supplemental motor area following sEEG implantation. Once the EZ is lateralized and localized, RSE can be treated with focal resection or ablation, and CC can be used in multistage epilepsy surgery for children with drug-resistant epilepsy [18, 19]. In a retrospective study of 10 patients with a focal EZ and presenting with RSE, all were treated surgically and without mortality [20].
The CC can be complete or focused to the anterior two-thirds and with an open technique following craniotomy or performed with minimally invasive MRgLITT [5, 6, 15]. Technique selection and extent of CC are based on patient age, baseline neurocognitive status, and surgeon preference [15]. Dallas et al. [15] reported that pediatric patients are better served by complete callosotomy owing to better rates of seizure control if patients have low neurocognitive status prior to surgery. A recent meta-analysis showed complete seizure freedom at a rate of 18.8% [21]. Parental satisfaction is seen in most cases secondary to functional improvements and seizure reduction [22].
While generally well tolerated, CC can result in disconnection syndromes which occur due to failure to transfer unilateral stimuli through the brain [23]. Other possible complications of CC include hemorrhage and infection, though these are rare. Disconnection syndromes following CC include supplementary motor area syndrome, hemispatial neglect, astereognosis, or alexia without agraphia, though these deficits are often transient and minimized by performing only a partial CC [23].
Epilepsy surgery should be considered in patients with focal drug-resistant epilepsy or NORSE with SRSE after the failure of medical management, defined as a trial of two antiseizure medications of appropriate mechanisms that do not result in seizure freedom and if no reversible cause of seizures is found [2, 3, 24]. We describe a case of NORSE with SRSE in a child with a challenging bilateral ictal pattern on scalp EEG and sEEG, with MRgLITT CC successfully lateralizing the EZ.
CC is often utilized for generalized drop attacks or tonic seizures or for nonlocalizable seizures, though should also be considered in a staged surgical approach for definitive focal epilepsy surgery. MRgLITT CC is minimally invasive and can lateralize the EZ when faced with bilateral homologous ictal patterns on scalp EEG and sEEG. In cases of drug-resistant epilepsy which is suspected to be focal due to semiology or other phase 1 presurgical testing, MRgLITT CC can be utilized in a staged surgical approach following failure of conventional medical management if bilateral interictal or ictal patterns are seen. Our case represents the first use of MRgLITT CC to lateralize a seizure focus in a child for reimplantation of sEEG electrodes in a staged surgical approach for definitive epilepsy surgery. We also demonstrate that MRgLITT may be useful in disrupting a seizure network to abort status in patients with NORSE.
The patient’s network was complex and incompletely treated due to the left inferior frontal contacts likely being associated with eloquent cortex. She was right-handed and could not cooperate with formal neuropsychological testing, functional MRI, or cortical mapping of her language cortex during her phase 2 evaluation due to her clinical condition. She suffered a post-operative intracranial hemorrhage after the reimplantation of sEEG electrodes, which did not require further surgery. She has intermittent seizures and is currently maintained with a vagus nerve stimulator (VNS), lacosamide, clobazam, cannabidiol, and perampanel. At 1 year follow-up, she is independently ambulatory, runs well, and speaks clearly. She does have an individualized education program (IEP) in school and intermittent seizures requiring titration of her medications and VNS settings. The family provided written consent for this case report.
CC: corpus callosotomy
EEG: electroencephalography
EZ: epileptogenic zone
ICU: intensive care unit
IV: intravenous
MRgLITT: magnetic resonance-guided laser interstitial thermal therapy
MRI: magnetic resonance imaging
NORSE: new onset refractory status epilepticus
RSE: refractory status epilepticus
sEEG: stereo-electroencephalogram
SPECT: single photon emission computerized tomography
SRSE: super refractory status epilepticus
KS, DM: Writing—original draft, Conceptualization, Writing—review & editing. RB, LS, SC, DS, MO, and RR: Writing—review & editing. AS: Writing— review & editing, Project administration. JR: Writing—review & editing, Supervision.
The authors declare that they have no conflicts of interest.
This case report was reviewed by the Indiana University Internal Review Board and approved with IRB protocol (22950).
Informed consent for authoring this case report was obtained from the parents of the patient.
Informed consent to publication was obtained from the parents of the patient prior to writing this case report.
The raw data that support the findings of this study are available from the corresponding author (Derryl Joseph Miller, miller89@iupui.edu) upon reasonable request.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Srilaxmi Vityala ... Swathi Nenavath
Joham Choque-Velasquez ... Alder Fernando Valenzuela-Rangel
Rene Ivan Gonzalez-Fernandez ... Jose Luis Hernandez-Caceres
Eva Žerovnik
Christine Walker, Chris L. Peterson
Swati Banerjee, Viktor Jirsa
Darrell O. Ricke
Fumiki Yamashita ... Mari Wataya-Kaneda
Jinwei Zhang
