Affiliation:
1Department of Physiology, Midnapore College, Midnapore 721101, West Bengal, India
Email: saptadip174@gmail.com; saptadip.samanta@midnaporecollege.ac.in
ORCID: https://orcid.org/0000-0001-6741-699X
Affiliation:
2Department of Psychology, Gordon F. Derner School of Psychology, Adelphi University, and Department of Biology, College of Arts and Sciences, Adelphi University, Garden City, NY 11530, USA
3Department of Pharmaceutical Sciences, College of Pharmacy, Southern University, Houston, TX 77004, USA
ORCID: https://orcid.org/0000-0001-9478-6427
Explor Neurosci. 2024;3:321–351 DOI: https://doi.org/10.37349/en.2024.00053
Received: March 20, 2024 Accepted: June 11, 2024 Published: August 23, 2024
Academic Editor: Ertugrul Kilic, Istanbul Medipol University, Turkey
The article belongs to the special issue Circadian Rhythm and Melatonin
There is a complex relationship between circadian rhythm dysfunctions and various psychiatric disorders. Circadian (~24 h) rhythms indicate the rhythmic change of different physiological activities in relation to the environmental light-dark cycle. Shift work, light exposure at night, and chronic and acute jet lag affect circadian rhythm that have a negative impact on psychological functions, and behaviors. Additionally, professional stress, mental instability, and social disintegration influence psychiatric disorders. PubMed/MEDLINE, Springer Nature, Science Direct (Elsevier), Wiley Online, ResearchGate, and Google Scholar databases were searched to collect relevant articles. Circadian rhythm disruption causes impaired neurotransmitter release, impaired melatonin and cortisol rhythm, metabolic dysfunctions, neuroinflammation, and neural apoptosis; collectively these factors influence the development of psychiatric disorders. Circadian dysfunction also alters the expression of several clock control genes in the mesolimbic areas that are associated with pathologies of psychiatric disorders. Additionally, chronotherapy and applications of anti-psychotic medicine can improve psychiatric diseases. This review focuses on the effects of circadian clock dysfunction on the vulnerability of psychiatric disorders and the implications of chronotherapy.
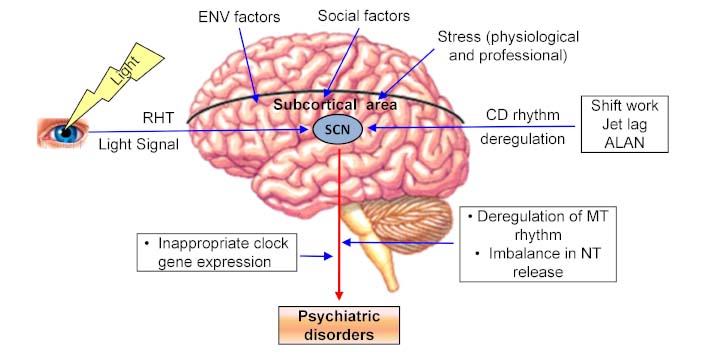
Different factors affect the circadian rhythm and also subucortical areas. Suprachiasmatic nucleus (SCN) acts as a master clock and sends output in different areas of the brain for the regulation of various physiological functions. Impairment of SCN activities and rhythmic gene expression promote psychiatric disorders. ALAN: artificial light at night; CD: circadian; ENV: environmental; MT: melatonin; NT: neurotransmitters; RHT: retinohypothalamic tract
Psychiatric disorders are serious problems throughout the world. The global scenario indicates that the mental illness burden is about 13.0% of disability-adjusted life-years (DALY) and 32.4% of years lived with disability (YLD) [1]. Psychiatric disorders affect cognition, emotional, and behavioral activities. Psychiatric disorders begin in early life and often appear as chronic recurrent diseases. There are different types of psychiatric diseases, including schizophrenia, attention deficit/hyperactivity disorder (ADHD), autism, major depressive disorder (MDD), bipolar disorder (BD), anxiety disorders, and seasonal affective disorder (SAD). Mental illness is detrimental to cognitive behavior, social activities, and economic performance. Psychiatric disorders have complex etiologies, and genetic defects, altered gene expression, mitochondrial dysfunction, and poor antioxidant activity are the influencing factors. Other factors, like environmental factors (antenatal maternal viral infection, fetal hypoxia due to obstetric complications, mother’s stress during fetal neural development, migration, urbanicity, social stress, and exposure to lead) and neuroinflammation are also associated with psychiatric diseases. Inflammatory mediators, particularly different cytokines alter neuroendocrine activity, synaptic plasticity, release of neurotransmitters (NTs) release, and monoamine metabolism, leading to the progression of mental illness [2, 3]. Some diseases may be linked with gender variation. The risk of autism is higher in males, while females have a higher rate of MDD, and anxiety disorders [4]. According to Patel and Kondratov [5], adverse circumstances, early childhood abuse, violence, poverty, and stress (physical, mental, and social) can promote psychiatric disorders. Currently, different psychotropic drugs are used for the treatment of schizophrenia, BD, major depression, and anxiety disorders. However, chronotherapy can improve psychiatric disorders.
Circadian rhythms are the rhythmic physiological manifestations along the solar day. These ~24-hour rhythms are controlled by the suprachiasmatic nucleus (SCN) of the hypothalamus. A complex relationship exists between circadian rhythms and mental health. Jet lag, shift work, and artificial light exposure at night promote circadian rhythm disruption. This dysfunction disturbs clock-controlled responses, such as the sleep-wake cycle cortisol and melatonin secretion, and core clock gene expression, resulting in mood disorders, depression, mania, and schizophrenia [6, 7]. The present review has focused on the relationship between circadian rhythm dysfunction and psychiatric disease, particularly schizophrenia, anxiety, depression, BD, MDD, autism, and SAD.
PubMed/MEDLINE, Springer Nature, Science Direct (Elsevier), Wiley Online, ResearchGate, Google Scholar databases, and others were thoroughly reviewed to search for relevant articles on SCN activity, circadian dysfunction, and psychiatric disorders. Different keywords like structure and functions of SCN, circadian rhythm dysfunction/chronodisruption, psychiatric disorders/mental illness, and circadian dysfunction and psychiatric diseases were used during the literature survey. The final version of this review has been prepared based on the search content.
Psychiatric disorders are characterized by significant disturbances in cognition, emotion, and behavior. Different cytokines, chemokines, and growth factors are prime components of neuroinflammation and these agents act as the pro-inflammatory and anti-inflammatory subsets. An imbalance in the expression of pro-inflammatory and anti-inflammatory agents potentially influences the development of various neuropsychiatric diseases [8]. Multiple sclerosis (MS) is an inflammation-mediated neurodegenerative disease. The risk of depression and anxiety is higher in patients with MS [9]. MS patients showing a relapsing stage exhibited elevated levels of inflammatory cytokines TNF-α, IL-1β, and IL-2 in the cerebrospinal fluid (CSF) [10]. Several areas of the brain are affected by various psychiatric diseases, and multiple factors are associated with these disorders (Table 1).
Primary symptoms, cause, and fundamental treatment strategies of common psychiatric diseases
| Psychiatric disorders | Symptoms | Affected brain area | Causes | Treatment |
|---|---|---|---|---|
| Schizophrenia | Hallucinations, delusions, disorganized thinking, avolition, diminished emotional expression, cognitive impairments | Frontal and temporal lobes, hippocampus, amygdala, and thalamus |
|
|
| Anxiety disorders | Chest pain, palpitations, respiratory difficulty, headache fear, tachycardia, shortness of breath, sweating | Amygdala, hypothalamus, locus coeruleus |
|
|
| Depression | Poor mental health, high suicide risk, decreased quality of life, drug addiction | Prefrontal cortex, hippocampus, amygdala, and Brodmann Area 25 |
|
|
| Major depressive disorder | Depressed mood, sadness, poor pleasure feeling, insomnia, excessive guilty feeling, hopelessness, problem to concentrate on work, suicidal thinking | Hypothalamus, limbic system, basal ganglia, and cerebellum |
|
|
DTNBP1: dystrobrevin binding protein 1; NRG1: neuregulin 1; COMT: catechol-O-methyl transferase; RGS4: regulator of G-protein signaling 4; GRM3: glutamate metabotropic receptor 3; DISC1: disrupted in schizophrenia 1; DAOA: D-amino acid oxidase activator; GABA: γ-aminobutyric acid
Schizophrenia is a chronic mental illness. The superior temporal gyrus and dorsolateral prefrontal cortex (PFC) are the most affected areas [11, 12]. This disease is characterized by hallucinations, delusions, disorganized thinking, avolition, diminished emotional expression, and cognitive impairments. In extreme cases, schizophrenia affects the patients’ livelihoods [13]. Schizophrenia patients show hallucinations through sensory modalities, particularly by hearing voices or noises. Delusions depend on beliefs and negative thoughts that are not associated with the patient’s culture. The patients exhibit social deficits, disorganized thoughts, speech-impaired emotional responses, and lack of motivation. There are learning restrictions and poor memory capacity among the sufferers [14]. Different cognitive tests like Stroop tests, Wisconsin card sorting tests, and verbal learning tests exhibit poor results [15]. Abnormalities occur in the frontal and temporal lobes, hippocampus, amygdala, and thalamus. Moreover, impaired secretion of dopamine and serotonin is also associated with this disease. Many genes are associated with various mental diseases. More than a dozen genes increase the risk of schizophrenia. Among these, mutation in the dystrobrevin binding protein 1 (DTNBP1), neuregulin 1 (NRG1), catechol-O-methyl transferase (COMT), regulator of G-protein signaling 4 (RGS4), glutamate metabotropic receptor 3 (GRM3), disrupted in schizophrenia 1 (DISC1), and D-amino acid oxidase activator (DAOA) genes increase the susceptibility of schizophrenia [16]. Neuroinflammation plays a vital role in schizophrenia pathology [17]. Results of a meta-analysis study indicated that patients with schizophrenia have increased levels of IL-17, IL-23, IL-6, TNF-α, and soluble IL-2 receptor (sIL-2R) [18, 19].
Microglia are activated by damage-associated molecular patterns, ionized calcium-binding adaptor molecule 1, and heat shock protein 70. Activated microglia produces pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) and reactive oxygen species (ROS). Microglia also induce the expression of inducible nitric oxide synthase (iNOS) for the synthesis of nitric oxide (NO). Expressions of advanced glycation end-product receptors in the neuronal microenvironment also occur during pathogenesis. Collectively, all these effects promote atrophy of the astrocytes and apoptosis of the neural cells, resulting in behavioral and cognitive deficits in schizophrenic patients [20]. Antipsychotic medication like clozapine is used for the treatment of schizophrenia. Moreover, counseling and rehabilitation can give positive results [21]. Cognitive therapy is applicable for the improvement of schizophrenia.
Anxiety disorders have complex features. Most of the patients show somatic complications, such as chest pain, palpitations, respiratory difficulty, and headache. The impaired activity of γ-aminobutyric acid (GABA)ergic neurons and functional defects in the amygdala-hypothalamus-central grey matter-locus coeruleus circuit are associated with anxiety disorders [22]. Panic disorder is a class of anxiety disorders, which is accompanied by fear, tachycardia, shortness of breath, and excessive sweating [23, 24]. People with panic disorder may restrict their activities to avoid situations where they feel panic. They sometimes avoid crowds, traveling, and elevators. Decreased levels of cortisol and prolactin may be the causative factors [25]. Excessive worry and fear have been observed in generalized anxiety disorder, which stimulates the sympathetic nervous system [26].
Social anxiety disorder is a chronic mental condition where fear and anxiety appear when an individual is surrounded by people. It is a common type of anxiety disorder. Affected individuals are feeling shyness, discomfort, and embarrassment in certain situations. They commonly try to avoid public addresses. They feel nervous and worried about teasing, rejection, and humiliation. Their voice becomes shaky. Therefore, they have poor social skills, difficulty in making social relationships, and low academic and employment achievements. Victim persons are feeling fear when they are scrutinized, evaluated, or judged by others. They have difficulties during answering a question in a class, job interview, and speaking in a public interview. Primarily, social anxiety disorder has a familial history and appears as an inherited trait. In this case, hyperactivity occurs in the amygdala. Cognitive behavioral therapy (CBT), antidepressants like selective serotonin reuptake inhibitors (SSRIs), beta-blockers, and anxiolytic drugs like benzodiazepines give positive results against social anxiety disorder [27].
Phobias are a type of anxiety disorder that causes an irrational fear from a situation, living system, or object. Specific phobia arises from the realization of a specific object, an event, or a situation. Social phobia is a fear of public humiliation. Agoraphobia creates a fear of a situation from which it would be difficult to escape. Phobias are also divided into different subtypes like personalized (blood, injection, injury), animals (spiders, insects, snakes, dogs), natural (flood, storm, earthquake, height), and situations (airplanes, elevators, enclosed places). Affected individuals show sweating, abnormal breathing, tachycardia, headache, trembling, chest pain, and dry mouth. Antidepressants like SSRIs, beta-blockers, benzodiazepines, and monoamine oxidase (MAO) inhibitors can be used for treatment [28].
Posttraumatic stress disorder (PTSD) shows traumatic episodes and emotional numbness after any exposure to environmental situations that remind the trauma and affect sleep quality [29]. Various causes, like accidents, disasters, physical or sexual assault, and terror attacks can initiate PTSD. The individuals show unnecessary fear, nightmares, severe anxiety, feeling tense, and depressing thoughts. In anxiety and PTSD, functional defects occur in the hypothalamus and amygdala [30, 31]. Abnormal secretions of cortisol and norepinephrine are also involved in these disorders. Moreover, different psychological factors like negative appraisals, fatalistic beliefs, early childhood traumas, lack of social support, limited coping skills, and poor self-capacities affect traumatic disorders. Hayes et al. [32] reported that CBT can improve the conditions of post-traumatic disorder. Social support, education, and guidance can improve the symptoms. The application of SSRIs may give positive results.
Mood disorders predominantly affect the emotional state of the person, which is indicated by depression, and irritation. Moreover, abnormalities in emotional behavior cause physiological changes, such as disturbance in the sleep-wake cycle, loss of appetite, poor energy output, and cognitive impairment. MDD and BD are the most common mood disorders.
Depression is considered a psychological symptom of stress, which is a highly comorbid disorder. This condition causes upsetting of mental health that affects the individual’s life. Depression is known as one of the most common psychiatric conditions where the risk of suicide, drug consumption, and healthcare costs, are high, and their quality of life is poor. Affected persons are facing social and economic problems [33]. Depression commonly occurs when insufficient concentrations of NTs are present in monoaminergic synapses. Impaired secretion of noradrenaline (NA) and 5-hydroxytryptamine (5-HT) also known as serotonin can promote depression. The exogenous depressive agent like reserpine depletes monoamine NT and tends to increase depression. Tyrosine hydroxylase is an essential enzyme for NA synthesis. Mutation in chromosome 11 causes defective expression of tyrosine hydroxylase, resulting in low levels of NA. On the other hand, dysfunction in the serotoninergic neurons in different areas, including the PFC, hippocampus, amygdala, and Brodmann area 25 causes exaggeration of depression. Neuroinflammation also decreases serotonin levels by activating the kynurenine pathway. This activity modulates the function of the N-methyl-D-aspartate (NMDA) receptors and diminishes serotonin levels, leading to the progression of depressive disorder [34]. Moreover, the hypothalamic-pituitary-adrenal (HPA) axis is also involved in pathophysiology of depression [35].
Stress response influences focal ischemia and glial activation that induces the expression of cytokines (TNF-α, IL-1β, and IL-6) for the advancement of depression [36]. The stress response also activates NMDA receptors that transiently increase the expression of iNOS for the synthesis of NO. The subsequent effect is the induction of cytokine release. Experimentally, lipopolysaccharide (LPS) is the potent inducer of TNF-α expression. The inflammatory response also affects NT metabolism, neuroendocrine function, and neural plasticity. Synthesis of dopamine and serotonin is notably decreased in depressed individuals [36]. Pharmacotherapy and CBT are the common treatments for depression and anxiety [37]. MAO inhibitors such as clorgyline and deprenyline decrease the breakdown of monoamines and act as the antidepressants. The reuptake of monoamine from the synaptic terminal is inhibited by reuptake inhibitors (imipramine and amitryptiline) that can improve depressive effects. Moreover, antidepressants downregulate the expression of presynaptic monoamine receptors. Natural products are being used as alternatives to pharmacotherapy for the management of various diseases, including depression and anxiety [38]. Among them, natural compounds flavonoids are effective in decreasing depressive symptoms in experimental models, possibly through the brain-derived neurotrophic factor (BDNF) expression, activation of monoaminergic systems as well as antioxidant effects [39].
It is characterized by continuously depressed mood, sadness, psychomotor agitation, poor pleasure feelings, insomnia or hypersomnia, excessive guilty feelings, hopelessness, decreased ability to concentrate on work, and feelings of worthlessness and thoughts of death. Some physiological changes also occur; these include sleep disturbance, poor memory, loss of appetite, weight loss, decreased energy output, and slowed motor movements [40]. Neuroinflammation progresses MDD. High levels of cytokines, such as IL-17, IL-23, IL-6, TNF-α, sIL-2R, and IL-1 receptor antagonist (IL-1RA) were observed in patients with MDD and BD [18, 41]. However, macrophage migration inhibitory factor (MIF) exerts protective measures against MDD [41].
BD shows extreme mood swings behavior. Episodic mania and depression along with relative periods of healthy mood (euthymia) are the common features of BD that appear cyclically with a period of interval. BD is divided into four categories on the basis of symptoms. Bipolar I disorder shows severe manic symptoms that last for at least 7 days. Depressive episodes also occur that continue for 2 weeks. Bipolar II disorder exhibits a pattern of depressive episodes and hypomanic episodes. The third type is cyclothymic disorder, where recurring hypomanic and depressive symptoms appear but do not last for a long time. If the symptoms do not match with these three categories then the disorder is specified as other or bipolar related disorders.
Sometimes BD shows both features of bipolar I and II. This is also called mixed affective BD. In this type, depression and mania or hypomania occur at the same time or very quickly after each other. The mixed affective states are more severe and very difficult to treat. Imbalance occurs in both the catecholaminergic and cholinergic systems. The mixed affective disorder is also associated with circadian disruption. Circadian rhythm irregularities cause stressful life, sleep problems, arrhythmic melatonin, and cortisol secretion. Thus, circadian rhythm dysfunction is a major contributor to affective symptoms [42].
Mania is a typical feature of BD, which is characterized by insomnia, and irritability. Mania often exhibits intrusive, impulsive, disinhibited behaviors, and poor judgment. Social jet lag promotes mania, but not depression in BD patients [43]. BD patients show sleep problems. The patients may suffer from thought difficulties and jump rapidly from one idea to another. BD seems to be a genetic disorder and exhibits heritability characteristics [44]. The affected areas are the basal ganglia and cerebellum. Abnormal activities of noradrenergic, serotoninergic, and GABAergic neurons are linked with BD. Mice model study revealed that low expression of dopamine transporter exhibits mania-like behaviors; thus, dopaminergic signaling is associated with BD [45].
Unipolar disorder shows one or more episodes of moderate to severe depression. Mania is a common phenomenon in BD. However, in unipolar disorder, there is a lack of alternative episodes of mania and depression. Affected individuals show sleep problems, feelings of sadness, inability to experience pleasure, lack of concentration in activity, change in appetite, body weight, and suicidal thoughts. Commonly, social, familial, and neurological factors are involved in this disease. Unipolar disorder is qualitatively different in etiology from BD. BD has a stronger genetic basis than unipolar disorder. An imaging study revealed that reduced blood flow occurs into the cerebral cortex, particularly in the PFC, and corpus callosum. Impaired metabolism was observed in the PFC, temporal cortex, amygdala, and basal ganglia. Low levels of dopamine and NA decrease the activity of the mesolimbic area. Decreased level of serotonergic regulation is also involved in the pathogenesis. CBT, SSRIs, tricyclic anti-depressants, and MAO inhibitors can be used for treatment purposes [46].
Autism spectrum disorder (ASD) develops in early life, mostly in childhood. Affected individuals show intellectual and adaptive deficits. They feel difficulties in social communication and social interaction. Moreover, they have poor interest in activities and weak verbal and non-verbal communication. There is no definite cause of ASD. It is mostly idiopathic and multiple factors are associated with this disease. In some cases, genetic factors are involved. Single nucleotide variants and copy number variants are considered as the genetic problems [47]. Epigenetic changes increase the rate of abnormal DNA methylation, which promotes ASD. Older fathers carry multiple imprinted gene loci that are susceptible to epigenetic changes [48]. Animal studies revealed that abnormal nutrition, stress, and transplacental psychiatric drugs affect GABAergic, dopaminergic, serotonergic, and glutamatergic neurotransmission and hamper neurodevelopment in fetal life. Collectively, these factors promote ASD [49]. Singh et al. [50] reported that perinatal brain injury, especially cerebellar injury, is a contributing factor in ASD development. There is no curative treatment for ASD. However, behavior and communication therapy, speech therapy, and sensory integration therapy are effective for the treatment of ASD. These therapies increase social activity, and language skills and decrease behavioral problems, aggressiveness, and agitation. The goal of the treatment is to make the children functional and independent and to improve their quality of life. There is no specific medication. However, some medicines can improve certain symptoms. Neuroleptics (pimozide and haloperidol) are an effective choice against behavioral problems. Risperidone decreases aggressiveness and agitation. Antidepressant drugs and psychotic drugs can improve behavior. SSRIs act as the mood stabilizers. Melatonin gives promising results in combating sleep problems [51].
ADHD is a specific type of autism. Significant loss of attention and impulsive behavior are the primary symptoms. Failure to self-identity, impaired regulatory systems and mind development, high levels of stress, and family problems can influence ADHD. Prenatal difficulties have a negative impact on this disease. Different areas are mostly affected; these include the frontal and temporal lobes, striatum, limbic system, cerebellum, and brain stem nuclei. Improper secretion of dopamine occurs in this condition. There is a genetic predisposition in the contributing list. ADHD has a high risk of inheritability. Genetic variation may contribute to ADHD. Single nucleotide polymorphism (SNP) has a role in the development of ADHD. Several genes such as DRD4 (dopamine receptor), DRD5, DAT1 (dopamine transporter), SNAP25 (gene for synaptosomal associated protein 25 kd), and COMT (catechol O-methyl transferase) are associated with this disorder. Chromosomal abnormalities (sex chromosome, specifically, X chromosome) may also be involved. Various extrinsic factors like smoking, alcoholism, poor nutrition, and exposure to carbon monoxide (CO), lead, and polychlorinated biphenyl (PCB) during pregnancy, excessive mental stress and obesity of the mother, and low birth weight of the child have a role in the development of ADHD [52]. Different therapies can improve the symptoms of ADHD. CBT (training for social skills, function, and cognitive development) is a common psychotherapy, which is effective against ADHD without any medication. Pharmacotherapies are used for symptomatic treatment. There are three types of medication. These include stimulants (methylphenidate, dexamethylphenidate amphetamine, norepinephrine, and dopamine), non-stimulant (α-2 adrenergic receptor agonist, selective norepinephrine reuptake inhibitor), and antidepressants (bupropion, venlafaxine, desipromine). Additionally, supplementation of polyunsaturated fatty acids has some effective role [53].
SAD also called seasonal depression is a specific type of depression that is associated with seasonal changes. In most cases, SAD appears during early winter and goes away during the sunnier days of spring and summer. Some people show the opposite effects. Symptoms start during spring or summer. The common symptoms are feeling sad, depression, anxiety, restlessness, sleep problems, appetite changes, weight gain/loss, loss of interest in activities and enjoyment, difficulty to concentrating on work, feeling hopeless, worthless, or guilty, and suicidal thinking. Misalignment of circadian rhythm due to changes in the duration of the light period alters the melatonin output and sleep properties, resulting in the progression of SAD [54]. In addition to sleep disorders, abnormalities in the diurnal rhythm of core body temperature, impaired cortisol, and melatonin secretion are the contributing factors [55]. Moreover, genetic variations in the endogenous circadian clock are also linked with SAD [56, 57].
Impaired serotonergic activity with seasonal variation intensifies SAD. Animals and normal humans exhibit altered serotonin activity across the seasons. Sunlight regulates the serotonergic pathway. A lack of sunlight during winter decreases serotonin levels, leading to further amplification of depression. Human post-mortem samples of the hypothalamus showed considerable seasonal variations in serotonin levels. The minimum levels were found during the winter months of December and January. This effect probably alters eating behavior, carbohydrate craving, and body weight [58].
Tryptophan is the precursor of serotonin. There is a seasonal variation in tryptophan metabolism. 5-hydroxyindole acetic acid (5-HIAA) appears during serotonin synthesis. High levels of 5-HIAA indicate weak serotonergic activity in the brain. Low levels of 5-HIAA occur in the spring season. The highest level of tryptophan occurs during April and May. Moreover, the tryptophan depletion rate is low during the summer season [58]. Johansson et al. [59] reported that serotonin-related polymorphisms cause SAD. Morning bright light therapy (BLT) is the primary treatment choice for SAD. Serotonin supplementation along with LT exhibits promising results against SAD. Serotonin receptor 5-HT2C agonists and SSRIs are also effective against SAD. Moreover, negative air ionization liberates charged particles in the sleep area, which is effective for the treatment of SAD [58].
The cortical and subcortical areas of the human brain make the networks for synchronization of neural activity. These networks are responsible for the operation of different tasks and cognitive control. Aberration in the brain networks is the neurological basis of psychiatric disorders [60]. The neuroimaging study revealed that affected areas in most of the psychiatric disorders are the dorsal anterior cingulate cortex (dACC) and anterior insula. These areas are responses to motivational demands and environmental constraints. The medial PFC, posterior cingulate cortex, precuneus, and lateral parietal cortex are also associated with psychiatric illness. Another important network is frontoparietal network, which comprises lateral prefrontal and intraparietal areas. This network is associated with cognitive tasks, attention, and behavioral activity [61]. Cognitive control is regulated by three distinct brain networks, including the salience network (SN), the fronto-parietal network (FPN: “central-executive”), and the default mode network (DMN). It is suggested that these three networks are associated with psychiatric disorders: the SN is linked with the anterior insula, dorsal anterior cingulate cortex, and subcortical nodes. This network is associated with reward processing regions. The dorsolateral PFC and posterior parietal cortex are the part of FPN. DMN is formed with the medial posterior cingulate cortex, ventromedial PFC, medial temporal lobe, and angular gyrus [60]. Segal et al. [62] studied the heterogeneity of gray matter volume (GMV) on 1294 cases of different psychiatric disorders (ADHD, ASD, BD, schizophrenia, and others). They concluded that person-specific deviations were possible and regional GMV were highly heterogeneous, affecting rate is < 7% of people. PFC dysfunction is implicated with MDD, BD, ADHD, and schizophrenia. Right and left lateral PFC, and dorsolateral PFC are mostly affected areas, and these regions are overlapped in different psychiatric disorders.
In humans, the limbic system is involved in the regulation of emotion and behavior. It consists of a group of nuclei. These include the cingulate gyrus of the cerebral cortex, the hypothalamus, the fornix, the hippocampus, and the amygdala. Different fiber tracts make the input and output connection with the limbic system. The limbic system is connected with the PFC and association cortex. There are reciprocal and interneuronal communications among the hypothalamus, prefrontal and association cortex, mamillary bodies, anterior thalamic nuclei, cingulate gyrus, entorhinal cortex, fornix, hippocampus, and amygdala. The cingulate gyrus is connected with the parahippocampal gyrus, and it runs along with the corpus callosum. The cingulate gyrus is interconnected with other areas of the limbic system. The main efferent projections move to the anterior thalamic nuclei. Its response regulates autonomic, somatic, or behavioral activities. The hippocampus is another important part, which receives inputs from the entorhinal area of the hippocampal gyrus, and septal nuclei. The outputs project to the entorhinal area, subiculum, and septal nuclei. The septal nuclei receive reciprocal inputs from the hippocampus via the fornix, amygdala, ventral tegmental nuclei, cingulate gyrus, hypothalamus, and preoptic region via the medial forebrain bundle. The efferent connections go to the hippocampus, ventral tegmental nuclei, habenular nucleus, lateral hypothalamus (LH), and preoptic region. Stimulation of the septal area is associated with pleasure feelings. Dopamine is the main NT in this area, which helps in pleasurable sensations. The amygdala receives inputs from sensory areas of the cerebral cortex, temporal cortex, insular parts of the cortex, thalamic nuclei, and ventromedial hypothalamic nuclei. The efferents move to the stria terminalis, anterior hypothalamus, lateral preoptic nucleus, LH, thalamic nuclei, basal nuclei, reticular formation, and parasympathetic cranial nerve nuclei. The amygdala regulates behavioral and autonomic functions. The insula is a part of the cerebral cortex and is associated with the frontal, parietal, and temporal cortex. The rostral end of the insula is part of the limbic system. The hypothalamus is a crucial part for emotional behavior and regulates visceral and somatomotor activity. Different types of stresses such as physical, physiological, mental, and social stresses are important factors in psychiatric disorders. The hypothalamus, amygdala, hippocampus, and PFC are implicated in the stress response and also associated with psychiatric disorders. Increased and sustained activity in the amygdala is related to depression and mental health.
Shift work and jet lag disrupt circadian clock functions in the SCN and hamper the functions of the HPA axis. Li and Androulakis [63] studied the effect of shift work and jet lag on the circadian clock and the activity of the HPA axis. They observed that light sensitivity varies from individual to individual. Male subjects and younger individuals have good light sensitivity, while female and elderly people are less sensitive to light. They also predicted that diversity and flexibility are varied along with altered light schedules among the individuals. Transient shift work and jet lag affect the functions of the HPA axis in all individuals. However, some individuals are well tolerated and resynchronize the SCN (core and shell)-HPA axis activity; others are less tolerable and show a negative impact. Stress response stimulates the HPA axis and increases plasma cortisol levels. The paraventricular nucleus (PVN) of the hypothalamus releases corticotropin-releasing hormone (CRH), which stimulates the adrenal cortex through pituitary ACTH. The stress response also activates the hippocampus and PFC. Glucocorticoid receptors are present in the hippocampus and induce stress-related activity. Kalsbeek and Buijs [64] reported that SCN also controls the activity of thyrotropin-releasing hormone (TRH), and gonadotropin-releasing hormone (GnRH) secreting neurons. These hormones regulate thyroid and gonadal functions.
On our planet, all organisms are adapted to the 24-hour light-dark cycle, which is familiar as the circadian (circa = about; dies = day) rhythm. Many physiological and behavioral activities are maintained by the circadian rhythm (Figure 1). Endogenous physiological events are rhythmically coordinated by the internal clock. Hypothalamic SCN acts as the master clock that synchronizes with the environmental light-dark cycle. SCN receives impulse (photic signal) from the non-visual intrinsically photoreceptive retinal ganglion cells (ipRGCs) through the retinohypothalamic tract (RHT) (Figure 1). The ipRGCs express blue light (~480 nm) sensitive photopigment, known as melanopsin [65–67]. On exposure to light, ipRGCs propagate signals to the SCN through the RHT, which releases glutamate as an excitatory NT. Another NT is pituitary adenylate cyclase-activating polypeptide (PACAP). Glutamatergic and PACAPergic neurons are co-localized with GABAergic inhibitory neurons. Light-induced activation of the SCN increases Ca2+ influx and activates the intracellular signaling cascade [68].
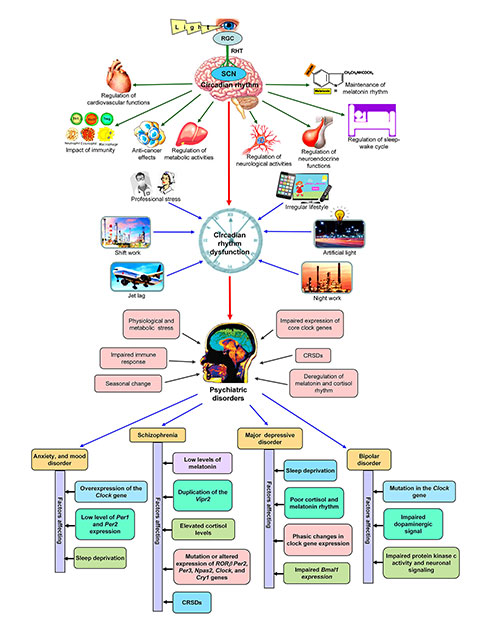
The relationship between circadian disruption and promotion of psychiatric disorders. The upper portion of the figure represents the regular functions of circadian rhythm and the lower portion of the figure shows how circadian dysfunction is associated with psychiatric diseases. CRSDs: circadian rhythm sleep disorders; RGC: retinal ganglion cells; RHT: retinohypothalamic tract; SCN: suprachiasmatic nucleus; Vipr2: vasoactive intestinal peptide receptor 2
SCN also receives non-retinal inputs. These projections come from the median raphe nucleus and intergeniculate leaflet (IGL) of the thalamus via the geniculohypothalamic tract. IGL neurons convey both photic and non-photic information to the SCN. These connections release GABA, neuropeptide Y (NPY), enkephalin, and neurotensin as the NT. The neurons from the median raphe nucleus are exclusively serotonergic. These inputs reset the SCN clock in response to behavioral and locomotor activity [69]. Ni et al. [70] reported that SCN receives non-photic inputs from locus coeruleus and periaqueductal gray in tree shrews. The SCN also receives projections from the ventromedial hypothalamus (VMH), arcuate nucleus (ARC), medial preoptic area (MPOA), paraventricular thalamus (PVT), and PVN of the hypothalamus [71]. The neurons of this projection release cholecystokinin (CCK) as a NT [72]. The vasoactive intestinal peptide (VIP)ergic and CCKergic secondary projections may be associated with motivational and behavioral activity. Dopaminergic neurons from the ventral tegmental area (VTA) send the impulse to the SCN and regulate photoentrainment and feeding-related activity [69].
Another important factor is steroid hormones. Different steroid hormones (testosterone, dihydrotestosterone, and estradiol) can cross the blood-brain barrier. The SCN expresses androgen receptor (AR), and estrogen receptors (ER) α and β [69]. ARs are expressed in the ventral SCN core. Testosterone/dihydrotestosterone interacts with ARs and then binds with androgen response elements on several core clock genes. This association also occurs on the negative regulator of Per1. Regulation of androgen-mediated transcriptional activity occurs in to dose-dependent manner [73]. ERs appear in the SCN shell and ERβ is predominant. Estrogen-ER complexes bind with estrogen response elements on the core clock genes, particularly Per2, and circadian locomotor output cycle kaput (Clock) [74, 75]. Thus, humoral factors appear in the SCN and regulate the expression of core clock genes.
SCN sends output signals to the hypothalamus, brainstem, pituitary gland, pineal gland, locus coeruleus, thalamic PVN, and other areas of the brain. SCN secretes a variety of NTs, including acetylcholine (Ach), glutamate, NPY, serotonin, norepinephrine, VIP, arginine vasopressin (AVP), GABA, and gastrin-releasing peptide (GRP). These NTs regulate the sleep-wake cycle, neuro-endocrine functions, melatonin release, and others. The rhythmic expression of clock genes controls the SCN-mediated functions. Arrhythmic expression of clock genes is associated with a variety of pathologies, including cancers, neurodegenerative diseases, and psychiatric disorders [68, 76].
The SCN sends the VIP, AVP, and prokineticin 2 (PK2)-expressing neurons to the adjacent hypothalamic and thalamic nuclei. The projections move to the subparaventricular zone, PVN of the hypothalamus, dorsomedial hypothalamus, VMH, anterior hypothalamus, posterior hypothalamus, ARC, ventromedial preoptic nucleus, ventrolateral preoptic nucleus, MPOA, periventricular nucleus, lateral habenula, lateral septum, stria terminalis, organum vasculosum of the lamina terminalis [69].
GABAergic neurons from the SCN to the dorsomedial hypothalamus inhibit the activity of sleep-promoting ventrolateral preoptic neurons. Glutamatergic projections to the dorsomedial hypothalamus activate wake-promoting hypocretin neurons in the LH. Hypothalamic regions directly or indirectly activate the reticular activating system and cerebral cortex to sustain wakefulness. Thus SCN regulates the sleep-wake cycle. Impairments in SCN activity promote sleep problems. Hypophagic or hyperphagic responses sometimes appear in psychiatric disorders. There is a relationship between the SCN and feeding activity. The SCN-ARC circuit may regulate NPY and agouti-related peptide release. The SCN projections also regulate leptin receptor expression. These activities have an impact on feeding behavior [77]. Light-dark cycle has a role in aggressive behavior. The SCN-VIP neurons send impulses to the GABAergic neurons of the subparaventricular zone that is connected with VMH. The circadian active phase increases aggressiveness. However, the inactive phase reduces the activity of the vesicular GABA transporter and decreases aggressive behavior [78]. Thus, circadian disruption may increase aggressive behavior. The SCN is indirectly connected with different regions of the brain that regulate arousal, motivation, mood, and behavior. Projections to the VTA, locus coeruleus, dorsal raphe nucleus, and nucleus accumbens via the dorsomedial hypothalamus, lateral habenula, MPOA, and thalamus regulate different neurological activities [79–81]. These connections may be associated with behavioral activity.
The SCN secretes different factors that can pass through the mesh capsule and act on the surrounding regions. These factors also enter into the CSF through the third ventricle. These diffusible factors include VIP, AVP, GRP, PK2, transforming growth factor-α (TGF-α), and cardiotrophin-like cytokine (CLC). The SCN can also release these factors to the portal system that is directly connected to the vascular bed in the organum vasculosum of the lamina terminalis. CLC receptors are located on neurons adjacent to the third ventricle and passes to the CSF. TGF-α receptors are present on the PVN that is associated with neuroendocrine functions. These factors show paracrine effects to the target regions of the brain [69]. These diffusible factors are rhythmically appeared and regulate the functions of the hypothalamus and other brain regions. Circadian dysfunction alters the activity of the SCN and may hamper the secretion of these factors. Thus, there is a chance of disorganized brain functions.
The molecular activity of the circadian clock is operated through transcriptional-translational feedback loops (TTFL). Different gene products of the circadian clock operate the TTFL process. Two proteins, CLOCK and brain and muscle ARNT-like protein 1 (BMAL1) form heterodimers. CLOCK-BMAL1 complex interacts with DNA at the E-boxes and upregulates the transcriptional activity of Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry 2) genes. PER and CRY proteins accumulate in the cytoplasm and translocate into the nucleus followed by the formation of heterodimer. PER-CRY heterodimer represses CLOCK-BMAL1-mediated transcriptional activity. Takahashi suggested that in mice, CLOCK-BMAL1-induced activation appears in the early morning but the PER-CRY complex exerts repressive effects in the evening/night [82]. On the other hand, reverse erythroblastosis viral oncogene homolog α (REV-ERB α) and RORα show contrastive effects. REV-ERB α promotes Bmal1 expression, whereas RORα represses Bmal1 transcription [83].
The risk of artificial light exposure at night is high in shift work, night work, social and acute jet lag. Light exposure at night causes phase shifting and alters the expression of core clock genes. Aberrant light exposure causes circadian clock dysfunction that disrupts different physiological rhythms, such as the sleep-wake cycle, cortisol, and melatonin secretion [84]. SCN regulates melatonin secretion from the pineal gland during normal photo-periodic activity. Exposure to light at night suppresses melatonin release. In the absence or low concentration of melatonin at night, cells switch on their activities in “day-mode” instead of “night mode”. The results are deregulation of gene expression, metabolic dysfunction, poor endogenous protective activity, and impaired behavior of the cells. Secretion of glucocorticoid occurs in the circadian rhythm by regulating the activity of the HPA axis. Glucocorticoid peak appears in the morning just before awakening and decreases throughout the day [85]. Circadian rhythm dysfunction alters the pattern of glucocorticoid release. Glucocorticoid regulates several physiological functions and is also regarded as a stress hormone. Dedovic and Ngiam [86] reported that impaired glucocorticoid secretion exaggerates MDD. Another report suggested that light-induced dysfunction of the HPA axis causes sleep disorders and increases the prevalence of cortisol-associated mood disorders [87]. Exposure to light at night disturbs the functions of ipRGC projections to the brain and may affect mood [88]. Melanopsin knockout mice have no impact on depressive responses on exposure to light at night [89].
Shift work, night work, acute and chronic jet lag, and exposure to artificial light at night are the intensive factors for circadian rhythm disruption (Figure 1). Light pollution is a serious issue in different metro cities of various countries. In recent years, many people are involved in shift work and night work. They are also facing jet lag problems. These people are in the risk zone for the development of psychiatric disorders. There is a close relationship between circadian rhythm dysfunction and psycho-behavioral disorders. The expression of core clock genes is under the control of circadian rhythm, while circadian rhythm dysfunction causes physiological, metabolic, and behavioral problems, resulting in the progression of psychiatric disorders (Figure 1). Circadian rhythm dysfunction promotes sleep disorders and alters the biomarkers of different physiological rhythms. Sleep problems appear as an early symptom of psychiatric disorders. Circadian rhythm disruption deregulates melatonin rhythm and sleep-wake cycle, which are associated with different psychiatric disorders, including SAD, BD, unipolar disorder, mood problems, schizophrenia, ASD, and ADHD (Figure 1) [90–92].
Schizophrenia is a well-known mental disorder with some symptomatic features, including delusions, hallucinations, movement disorders, anhedonia, alogia, and cognitive failure. Circadian rhythm sleep disorders (CRSDs) can promote schizophrenia [93, 94]. A report is also available on the arrhythmic expression of genes in post-mortem brain tissues of schizophrenic patients [95]. Monteleone et al. [96] reported that disruption of melatonin rhythm is associated with schizophrenia. Circadian rhythm disruption and phase shifting can uncouple the melatonin rhythm with the environmental light-dark cycle, leading to sleep disorder and the progression of schizophrenia [97]. Elevated cortisol level was observed in schizophrenic patients, which occurs due to hyperactivity of the HPA axis and also indicates the physiological stress during schizophrenia [98]. A specific gene, early gene growth response 3 (Egr3) is associated with circadian disruption and schizophrenia [99]. Egr3-deficient mice showed the characteristics of schizophrenia [100]. Mutation or altered expression of RORβ, Per2, Per3, Npas2, Clock, and Cry1 genes can develop schizophrenia [101, 102]. VIP is an essential NT in the CNS, particularly in the cortical interneurons. Abnormalities in VIP levels or its functions promote schizophrenia. Reduction in VIP levels in the prefrontal and orbitofrontal cortices occurs in schizophrenia. Despite VIP, the activity of VIP receptors is also important. Vasoactive intestinal peptide receptor 2 (VIPR2) knockout mice showed impaired hippocampal-associative memory deficit, irregular activity of the HPA axis, and cortisol secretion [103]. Alternatively, Duplication of the Vipr2 gene increases the risk of schizophrenia [104]. This duplication increases the expression of VIPR2; the result is the exaggeration of VIPR2 signaling and increased cAMP levels at the neuronal cells. Thus, VIPR2 signaling has a role in the pathogenesis of schizophrenia. These effects can progress schizophrenia and VIPR2 can be the potential target for the development of anti-psychiatric drugs.
Shift work influences sleep problems, anxiety, and mood changes. Imposed of night shift work to the day shift workers promotes sleep problems and anxiety [7]. Jet lag can influence the appearance of sleep disruption, anxiety, and dysphoric mood [105]. Nurses are facing psychiatric problems as they follow rotating shift schedules. They showed higher scores in anxiety studies when measured on the Hospital Anxiety and Depression Scale [106]. Animal experiments support the link between circadian disruption and anxiety. Anxiety-like behavior appeared when the adult rats were kept in constant light [107]. Genetic study indicates that improper expression of core clock genes has a relation to anxiety-like disorders. Overexpression of the Clock gene increases anxiety symptoms and ClockΔ19 mutation in mice decrease anxiety. Alternatively, anxiety-related problems are more common in Per1 and Per2 deficient mice. Suppression of Per1 and Per2 expression in the nucleus accumbens causes anxiety [108]. Disruption of circadian rhythms by exposure to constant light (20 h light-dark cycle) causes shortening of dendritic length and poor complexity of neurons in the prelimbic PFC in mice; the result is the exaggeration of anxiety [109].
Deregulation of the circadian rhythm increases the risk of MDD, which is characterized by feelings of sadness and irritability, sleep deprivation (SD), loss of sexual desire, poor appetite, slowing of speech, and suicidal thoughts [110]. Several studies indicated a clear relationship between shift work and depression [111–113]. A meta-analysis study was done by Lee et al. [112], and they reported that night shift work increases a 40% risk of depression compared to daytime workers. Ohayon and Hong [114] reported that the prevalence of MDD is more common in shift workers. A cohort study on night shift workers in Brazil indicated that females are mostly affected by MDD in comparison to males [115]. SD has an impact on MDD. The phase of rapid eye movement sleep increases due to decreased latency, whereas there is a marked deprivation in slow wave sleep. SD, particularly slow-wave sleep disrupts the secretory pattern of melatonin and cortisol, and also alters (increases) nocturnal body temperature; these factors can advance MDD [116]. A marked reduction in the expression pattern (amplitude and phase) of core clock genes had been observed in the post-mortem brain sample of MDD patients [117].
Several experimental studies on animal models indicate the association between circadian rhythm disruption and depression. Bmal1-knockdown mice showed depressive behavior during the impaired light-dark cycle [118, 119]. Nighttime light (dim light) exposure induces depression. Exposure to light at night in female Siberian hamsters alters the expression of clock proteins and cortisol rhythm [120]. Female hamsters kept in experimental jet lag condition exhibit neuroinflammation, decreased density of the dendritic spine in the hippocampus, and symptoms of depression [121]. The application of TNF-α inhibitor is protective against depressive-like behavior [122]. Experimental studies on male and female mice established that circadian dysfunction is associated with depressive-like disorders [123]. In this experiment, light exposure at night decreased vascular endothelial growth factor A (VEGF-A) in the hippocampus in both male and female mice. On the other hand, VEGF receptor 1 (VEGFR1) and IL-1β expression increased in female mice, while BDNF expression decreased in male mice. Moreover, acute exposure to dim light at night caused circadian dysfunction and altered the expression of clock genes [123]. Constant light exposure to rats for 8 weeks showed an arrhythmic pattern of melatonin and cortisol secretion, and depressive behavior [107]. Application of melatonin receptors’ agonist (agomelatine) on rats restored melatonin and cortisol rhythms and decreased depressive symptoms after exposure to chronic constant light [124, 125].
Walker et al. [123] indicated that LT could improve MDD. Applications of LT, wake therapy (WT), and antidepressants, such as SSRIs, are effective for the treatment of depression [126]. Treatment with LT, SSRIs, and melatonin receptors agonists make the phase advancement of body temperature, melatonin, and cortisol rhythms that improve depressive symptoms [127–129]. WT increases the amount of slow-wave sleep and decreases REM sleep, which gives potential benefits against MDD [130].
Another psychiatric problem is BD, where mania and depressive mood have cyclically appeared with a definite interval. Jet lag-related circadian rhythm dysfunction is associated with BD [131]. The transmeridian journey from east to west causes depression, while west to east develops mania [132]. Meta-analysis of clinical studies indicated that circadian rhythm disruption promotes BD [133]. Bellivier et al. [134] reported that clock genes are associated with BD. Polymorphisms in clock genes may increase the possibility of BD development. Le-Niculescu et al. [135] had shown the relationship between polymorphisms in core clock genes and the development of BD. Mutation in the Clock gene promotes mania-like symptoms. ClockΔ19 mutant mice showed bipolar mania, and administration of dopamine improved mania-like behaviors [136]. Moon et al. [137] suggested that the determination of clock gene expression and cortisol rhythm can be considered as the biomarkers in BD patients. Stabilization of sleep and circadian rhythm effectively improve BD [134]. Like MDD, LT is also effective in BD [138]. Therapeutic administration of lithium counteracts the fast-running circadian clock in BD patients and stabilizes circadian rhythmicity, leading to improvement in BD symptoms [139]. A report from different literature surveys revealed that impaired protein kinase C activity alters the neuronal signaling in the frontal and limbic area of the brain, leading to BD [140]. Cry and Npas2 genes are associated with unipolar disorder. The delayed sleep phase is common in patients with unipolar disorder. Affected individuals show higher sleep latency, late sleep off, and longer duration of sleep time [141].
Children with ASD exhibit sleep problems and circadian rhythm dysfunction. Moreover, circadian dysfunction also promotes sleep disorders. So, there is a close relationship between sleep problems and ASD [142]. Prolonged sleep latency, frequent waking at night, alterations in sleep pattern (low levels of REM sleep and higher percentage of slow-wave sleep), unusual morning arousal, and reduction in total sleep time are common features of ASD [143, 144]. Abnormal cortisol levels, higher serotonin levels, and high levels of melatonin in the daytime are associated with ASD [145]. Melatonin has a positive role in the sleep cycle and also regulates the clock gene expression in the SCN. Circadian misalignment deregulates melatonin rhythm, which also affects SCN functions. Melke et al. [146] reported that low levels of serum and urinary melatonin were observed in ASD individuals. The PVN secretes CRH, which regulates the activity of the HPA axis. The PVN receives neural input from the hippocampus, amygdala, PFC, and SCN. Circadian dysfunction alters SCN activities and CRH release from PVN, resulting in abnormalities in cortisol rhythm [147]. Serotonin is essential for the development of the forebrain in the fetus. Synthesis of serotonin depends on the sufficient concentrations of tryptophan during the pregnancy period. Abnormal serotonin concentration is detrimental for the development of ASD. There is a relationship between disturbance in the serotonergic system and circadian dysfunction; the result is the progression of ASD [148]. Abnormalities in clock gene expression cause ASD. The timing deficit in ASD may be associated with the structural and functional activity of the clock-related genes [149]. Experiments on mice model systems showed that NPAS2 is involved in the regulation of REM sleep and total sleep time. Impairment in NPAS2 increases the risk of ASD [150]. Mutation in NPAS2 (cytosine/thymine), and PER1 (cytosine/guanine SNP rs885747; cytosine/adenine SNP rs6416892) have a role in ASD development. Substitutions of proline/alanine at amino acid 1228 in PER2 and arginine/glutamine at amino acid 366 in PER3 are related to the pathogenesis of ASD. The impaired function of RORα alters the expression and function of Bmal1, which might be associated with ASD [145, 151].
Circadian dysfunction and sleep disorders (short sleep period and evening chronotype) are associated with ADHD. Huang et al. [152] reported that circadian dysfunction affects dopamine levels and the development of dopaminergic neurons, leading to the progression of ADHD. Melatonin and cortisol are the most important factors for the regulation of neurophysiological activities. Arrhythmic secretion of melatonin and cortisol occurs in ADHD individuals. The peak of melatonin and cortisol decreases in ADHD [153]. SNP in CLOCK locus causes ADHD. Animal experiments revealed that the CLOCK gene is involved in circadian sleep disorder and ADHD [154]. Genetic variation in the 3՛ untranslated region (UTR) of CLOCK gene may increase the susceptibility to ADHD development [155]. Baird et al. [156] observed arrhythmic expression of BMAL1 and PER2 genes in the ADHD group.
SAD/winter depression is a recurring depressive behavior that occurs annually in the winter and spontaneously disappears in the spring/summer [54]. Seasonal change alters the natural light-dark cycle that causes SAD. In the North Pole region, short day lengths in winter can initiate dysthymia. Oppositely, prolonged daylight in the equator region during summer starts euthymia. SAD patients showed advanced sleep phase syndrome (ASPS) and delayed sleep phase disorder/syndrome (DSPS) [54]. In ASPS, the sleep episode starts earlier than the desired clock time, leading to early sleep onset and early awakening. DSPS occurs when the onset of sleep and awakening is delayed than the desired clock time. Seasonal changes affect the natural light-dark cycle, causing a phase shift of the circadian clock that alters NT release (particularly serotonin), functions of neural circuits, melatonin, and cortisol rhythm. Excess melatonin in winter increases sleepiness. The collective effects are mood-related problems. LT and antipsychotic drugs can improve SAD [157]. In humans, SAD can develop after the formation of the variant protein NPAS2 471 Leu/Ser [158]. Polymorphism of PER3 (P415A/H417R) is associated with advanced sleep phase and SAD [159].
Regulation of sleep-wake cycle is a very complex system. During the daytime, high levels of NTs decrease sleep pressure and maintain awaking state. At night, sleep pressure reaches maximum. Orexigenic peptide orexin and its receptors are involved in the regulation of the sleep cycle. The neurons of LH release orexin A and orexin B. These peptides stimulate cholinergic and monoamine (serotonin, dopamine, NA, and histamine) secreting neurons in the hypothalamus and the brain stem for arousal response and awakening activities [160]. During daytime SCN continuously activates the LH and maintains wakefulness. Monoaminergic pathways also inhibit the ventrolateral preoptic area (VLPO) for suppressing the sleep cycle. At night, VLPO secrets sleep-inducing agents, which inhibit monoaminergic pathways in the LH for inducing sleep. Circadian disruption affects these pathways and disturbs the sleep-wake cycle, leading to the progression of psychiatric disorders.
The SCN controls the activity of the pineal gland and regulates melatonin synthesis and secretion. Melatonin receptors are present in the SCN, hypothalamus, substentia nigra, hippocampus, cerebellum, VTA, and nucleus accumbens [161]. Melatonin has a crucial role in brain functions and shows a sleep-inducing effect. Circadian rhythm disruption hampers melatonin rhythm and also causes SD. SD and circadian rhythm disruption occur in schizophrenic patients. They showed impaired melatonin rhythm [162]. Similarly, Naismith et al. [163] reported that patients with MDD have low salivary melatonin. Thus, there must be a link among circadian rhythm disruption, impaired melatonin rhythm, sleep disorders, and psychiatric disorders. Patients with BD and MDD also showed sleep disruption that may intensify the psychiatric disorders. Impaired BDNF level is associated with psychiatric disorders. BDNF can induce spontaneous wakefulness, leading to impaired neural functions and the progression of BD and MDD [164]. Glucose is an essential metabolite for neural cells. SD also affects glucose metabolism in the PFC [165]. Thus, sleep problems and psychiatric disorders have a bidirectional relationship.
Shank proteins (plasticity-associated synaptic proteins) are involved in synaptic plasticity. These proteins follow a circadian rhythm. Light affects the expression of Shank proteins. Sarowar et al. [166] suggested that schizophrenia is associated with impaired expression of Shank3. Cell adhesion molecule neurexin promotes synaptogenesis, synaptic transmission, and regulation of sleep quality [167]. Defects in neurexin gene expression in circadian dysfunction cause ASD and schizophrenia.
Circadian rhythm critically regulates the HPA axis activity. In the gestational period, glucocorticoid crosses the placenta and regulates neural development in the fetus. Circadian dysfunction acts as a stressor, and prenatal stress disturbs the expression of core clock genes in the SCN. Animal experiments also revealed that stress response in mothers causes impaired expression of Clock and Rev-erb α genes in the peripheral tissues (adrenal and liver). Additionally, hyperactivity of the HPA axis reduces the sensitivity of negative feedback of glucocorticoids in adult mice. Thus, stress response or circadian dysfunction affects the HPA axis and alters glucocorticoid rhythm. These effects on mother may increase the risk of development of ASD in the child during the postnatal period [165]. Disruption of HPA axis activity advances MDD, BD, and schizophrenia [168].
Chronotype is a factor in psychiatric disorders. MDD, BD, SAD, and anxiety disorders are associated with evening chronotype [169]. Shift work, jet lag, and night work have detrimental effects on the evening chronotype. These events are also the cause of circadian misalignment. A diffusion tensor imaging study indicated that the integrity of white matter in the frontal and temporal lobe, cingulate gyrus, and corpus callosum in the evening chronotype has some differences compared to the morning chronotype [170]. These differences are probably associated with psychiatric disorders in the evening chronotype. Thus, there is a complex relationship among chronotype, circadian dysfunction, and psychiatric disorders.
NTs like serotonin, dopamine, and NA are involved in pathologies of psychiatric disorders [171]. Serotonin is essential for the regulation of circadian rhythm, mood, anxiety, and cognitive functions [172]. The serotonergic system also regulates the HPA axis and cortisol release. Disruption of circadian rhythm causes disintegration in serotonergic activity and neural functions, as well as melatonin and cortisol secretion, resulting in an increased risk of MDD [173]. Depression is also associated with impaired functions of GABAergic and glutamatergic systems [174]. Moreover, low levels of serotonin, dopamine, and norepinephrine are correlated with depression [175]. In schizophrenia, impaired working memory and cognitive functions occur due to a deficit of glutamatergic NMDA receptors in the pyramidal neurons of the PFC [176]. Inhibitory GABA signaling in the pyramidal interneuron network is essential for working memory. Impaired GABAergic transmission in this area causes working memory deficits in schizophrenic subjects [177]. Kumar et al. [178] reported that low levels of GABAergic activity increase dopamine levels, which deregulate the activity of the dopaminergic system, causing behavioral problems in schizophrenia [179]. Moreover, excess dopamine secretion causes the progression of schizophrenia. In ASD, an imbalance occurs between inhibitory NT GABA and excitatory NT glutamate [180]. The glutamatergic projections from the frontal area to the striatum of basal ganglia may increase motor activities in ASD [181]. Mutation in glutamatergic receptor genes GRIN2A and GRIN2B promotes ASD [180]. Abnormalities in dopamine, norepinephrine, and serotonin secretion are linked with ASD and are correlated with sleep disturbance, mood disorders, and behavioral problems [182, 183]. Marotta et al. [180] reported that ASD patients had reduced dopamine levels in the PFC and nucleus accumbens. Impaired dopamine secretion in the nigrostriatal pathway and mesocorticolimbic area causes motor dysfunction and behavioral problems in ASD subjects, respectively. Thus, evidence-based studies revealed that there is a complex relationship among circadian disruption, neural activities, neuroendocrine dysfunction, and psychiatric disorders.
Treatments of psychiatric diseases decrease symptoms and provide better quality of life. Applications of antipsychotic, antidepressant, and mood-stabilizing drugs, such as haloperidol, perazine, lithium, valproate acid, clomipramine, fluoxetine, and SSRIs give positive results against psychiatric diseases. Escitalopram, venlafaxine, benzodiazepines, and buspirone are the prescribed drugs for anxiety disorder [31].
SSRIs are the first-line drug choice for the treatment of depression, anxiety, and other psychiatric disorders. SSRIs block the reuptake of serotonin, resulting in slow clearance of this monoamine NT. SSRIs increase serotonin levels at the synaptic cleft and promote serotonergic activities in the brain. Elevation of extraneural serotonin concentrations facilitates the binding of serotonin with somatodendritic autoreceptors (5-HT1A) and the autoreceptors of the presynaptic end (5-HT1B). This binding effectively maintains serotonergic neurotransmission. The common SSRIs are paroxetine, mirtazapine, nefazodone, and venlafaxine [184]. MAO inhibitors and SSRIs are used for the treatment of depression. Sometimes, prolonged use of SSRIs increases the risk of obesity [184].
Köhler et al. [185] reported that anti-inflammatory agents give relief against depressive symptoms. SSRIs decrease IL-1β, IL-6, and TNF-α in the peripheral circulation [186]. Baumeister et al. [187] indicated that clomipramine and fluoxetine reduce IL-6, TNF-α, and IFN-γ levels. Application of TNF inhibitor in female Siberian hamsters prevents dendritic spine formation in the hippocampus and ameliorates depressive-like behavior [120]. However, anti-inflammatory drugs can be used as an adjuvant in association with antipsychotic drugs for the better treatment of psychiatric diseases [188]. SSRIs, serotonin and NA reuptake inhibitors (SNRIs), agomelatine (MT1 and MT2 agonists), and 5-HT2C antagonists improve MDD. They help in phase advancement that is effective in the treatment process.
The pharmacological applications of lithium chloride for the treatment of circadian disruption show a significant effect on the resynchronization of circadian rhythm by altering the activity of intracellular kinases, including glycogen synthase kinase-3beta (GSK-3beta). The regulation of the circadian clock at the molecular level reduces the symptoms of mania, and depression and improves behavioral activity [6]. Lithium-induced normalization of circadian rhythms is essential for the treatment of BD. Lithium slows down the circadian dysfunction and ameliorates the symptoms of BD [189].
Sleep problem is associated with psychiatric disorders. The application of melatonin improves sleep problems and increases sleep quality. FDA also approved melatonin agonists for the treatment of insomnia. The melatonin agonist agomelatine acts as a zeitgeber and helps to establish regular sleep cycle. Agomelatine also shows antidepressant properties [190]. Administration of agomelatine to rats prevents depressive-like behavior even after exposure to constant light [124, 125]. The application of agomelatine on human subjects improves the rest-activity cycle and sleep quality, leading to the improvement of depressive symptoms [191].
Other pharmacological agents, such as NO synthase 1 adaptor protein (NOS1AP), a specific anti-psychiatric drug, are applicable for the treatment of BD and schizophrenia [192]. The allelic variants of FK506-binding protein 51 (FKBP51) reduce depression and anxiety disorders [193].
Psychotherapy such as CBT can improve panic disorder, phobias, and major depression. This treatment regulates attitudes and behaviors and increases intellectual activity and cognitive response [194, 195].
Chronotherapy is based on the regulation of circadian rhythms. It refers to non-pharmaceutical treatment that depends on exposure to external environmental stimuli to regulate the biological rhythms [196]. Chronotherapy includes SD or WT, sleep phase advance (SPA) therapy, and LT or BLT [197]. Exposure to white light (intensity 2,000–10,000 lux) for 30–120 minutes per day is given in BLT. Most of the time, treatment duration is 2–4 weeks. LT is based on neurobiological principles, and it is the effective treatment choice for SAD [198]. Khalifeh [199] reported that BLT is the promising treatment choice for SAD. LT is also effective for the treatment of non-seasonal depression and mood disorders [200, 201]. LT is effective for the treatment of SAD and non-seasonal depression. Tao et al. [202] conducted a meta-analysis on randomized controlled trials (RCTs) of depression study. They analyzed 23 RCTs with 1120 participants. The result indicated that LT has significant effects to reduce non-seasonal depressive symptoms. Another study on 89 patients (age 60 years or older) with MDD showed improvements in mood, sleep quality, and melatonin levels after BLT [203]. Hizli et al. [204] compared the effects between BLT and BLT with SSRI (fluoxetine). They reported that the application of BLT with SSRI had no extra benefit on depressive symptoms and sleep quality. LT along with antidepressants in unipolar disorder patients is a better choice for the treatment. The application of LT with lithium is also effective in BD patients [200, 201]. BLT in the morning also promotes phase advancement and improves depressive symptoms. Randomized, placebo-controlled clinical trials revealed that BLT in the midday prevents the episode of depression in BD [138]. LT is an alternative choice for patients who refuse, resist, or cannot tolerate any antidepressant drugs [205].
SD/WT is a process where individuals remain awake for long periods (up to 36 h) to reduce depressive symptoms [206, 207]. WT gives quick response and better efficacy in reducing depressive symptoms [196]. The effect of WT is short lasting and there is a chance of relapse. Casher et al. [208] suggested that WT increases serotonin, dopamine, and norepinephrine levels in the CNS for the improvement of psychiatric problems. Applications of BLT, and WT therapy improve MDD. WT increases the levels of slow wave sleep and decreases the latency of REM sleep, which are effective for the treatment of MDD [7]. A comparative study on MDD patients where one group is taking a combined treatment of WT, LT, and sleep time stabilization and another group is performing exercise therapy; the result indicated that WT, LT, and sleep time stabilization give better results than exercise therapy [209]. Benedetti et al. [210] reported that WT in patients with BD with or without drug resistance history has a remarkable potentiality of therapeutic efficacy.
Acombined use of BLT, WT, and SPA is used in triple chronotherapy. This therapy can give rapid improvements in depressive symptoms [211]. Moscovici and Kotler [212] applied chronobiologic multistage intervention (CMI) by using LT, SD, and SPA on 12 patients with moderate to severe depression and observed significant improvements in depressive symptoms. Several authors also reported that triple chronotherapy shows promising results for the treatment of depression [211, 213]. Wu et al. [214] also reported that triple chronotherapy along with the application of sertraline and lithium is effective for the treatment of BD.
Another type of chronotherapy is dark therapy (DT), where patients are kept in a complete dark room at night. Sometimes, blue-blocking sunglasses are used to induce “circadian darkness” [215]. DT is effective for the patient with BD [216]. A clinical trial reported that DT and blocking of blue light exposure prevent bipolar mania [217].
Recently, gene therapy and stem cell therapy have been applied as newer approaches for the treatment of psychiatric diseases. Both these processes show significant success in mouse models for the treatment of different forms of mental illness. In humans, low levels of the p11 protein expression are associated with depression. p11 protein knockout mice also exhibit depression. Administration of an adeno-associated virus carrying the gene of the p11 protein into the nucleus accumbens restores the expression of p11 protein and reduces the symptoms of depression [218].
In recent years, circadian rhythm dysfunction is a common phenomenon in our modern society due to shift work, night work, exposure to bright light at night, and jet lag. Circadian rhythm misalignment adversely affects human health, particularly neural functions, neuroendocrine activity, and metabolic functions. Alterations of circadian rhythms are also associated with neurodegenerative diseases and psychiatric disorders, including schizophrenia, anxiety, depression, MDD, and BD. Circadian disruption deregulates the expression of core clock genes, melatonin and cortisol rhythm, activity of NTs, and function of different areas of the brain. Moreover, physical and physiological stress, neuroinflammation, and neurodegeneration also affect neuronal activity. Collectively, these effects promote psychiatric diseases. Resynchronization of circadian rhythms can improve the symptoms of psychiatric illness. Anti-psychotic drugs, CBT, LT, WT, and family support are beneficial for the treatment of psychiatric disorders. Finally, it can be concluded that public awareness about mental illness and circadian dysfunction is the forward step for combating psychiatric disorders. Activities at the personal level to maintain the circadian rhythm can give fruitful results in the near future.
5-HIAA: 5-hydroxyindole acetic acid
5-HT: 5-hydroxytryptamine
ADHD: attention deficit/hyperactivity disorder
AR: androgen receptor
ARC: arcuate nucleus
ASD: autism spectrum disorder
BD: bipolar disorder
BDNF: brain-derived neurotrophic factor
BLT: bright light therap
BMAL1: muscle ARNT-like protein 1
CBT: cognitive behavioral therap
CLOCK: circadian locomotor output cycle kaput
CRH: corticotropin-releasing hormone
CSF: cerebrospinal fluid
DT: dark therapy
ER: estrogen receptors
GABA: γ-aminobutyric acid
HPA: hypothalamic-pituitary-adrenal
iNOS: inducible nitric oxide synthase
ipRGCs: intrinsically photoreceptive retinal ganglion cells
LH: lateral hypothalamus
LT: light therapy
MAO: monoamine oxidase
MDD: major depressive disorder
MPOA: medial preoptic area
MS: multiple sclerosis
NA: noradrenaline
NMDA: N-methyl-D-aspartate
NO: nitric oxide
NPY: neuropeptide Y
NTs: neurotransmitters
PFC: prefrontal cortex
PTSD: posttraumatic stress disorder
PVN: paraventricular nucleus
REV-ERB α: reverse erythroblastosis viral oncogene homolog α
SAD: seasonal affective disorder
SCN: suprachiasmatic nucleus
SD: sleep deprivation
SNP: single nucleotide polymorphism
SPA: sleep phase advance
SSRIs: selective serotonin reuptake inhibitors
VIP: vasoactive intestinal peptide
VIPR2: vasoactive intestinal peptide receptor 2
VMH: ventromedial hypothalamus
VTA: ventral tegmental area
WT: wake therapy
SS: Conceptualization, Data curation, Writing—original draft, Writing—review & editing. DB: Supervision. The authors have agreed to the published version of the manuscript.
Dr. Blum is the inventor of the Genetic Addiction Risk Severity (GARS), USA and foreign patents and KB220 Patented products. The other author declare that there is no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Saptadip Samanta, Sk Asif Ali
Georges Maestroni
Utku Aykan ... Canan Uluoglu
Viktória Vereczki ... Ágnes Csáki
