Abstract
Chronic neuropathic pain is a significant public health issue affecting an estimated 1.5 billion individuals worldwide. The mechanisms underlying chronic pain are multifaceted and not fully understood. Chronic pain amplifies specific neural pathways through peripheral and central sensitization triggered by repeated exposure to noxious stimuli, ultimately resulting in physical and emotional pain. Traditional treatment options targeting these mechanisms, such as opioid and non-opioid analgesics, are associated with adverse effects, addiction, and suboptimal pain relief. Using psychedelics to treat chronic pain is an area of growing interest. While psychedelic substances, such as psilocybin, lysergic acid diethylamide, mescaline, and 3,4-methylenedioxymethamphetamine are primarily associated with recreational use or spiritual practices, emerging evidence suggests their potential therapeutic benefits for various mental health disorders, including chronic pain. Psychedelics alter pain perception by directly activating serotonin receptors, exerting anti-inflammatory effects, enhancing descending inhibition, opening a window of neuroplasticity, and facilitating synaptic remodeling. This review mainly elucidates the ongoing research regarding the psychedelic mechanisms of action, pharmacology, clinical applications, and therapeutic potential in treating neuropathic pain.
Keywords
Psilocybin, cluster headache, fibromyalgia, neuroplasticityIntroduction
Psilocybin, a naturally abundant tryptamine alkaloid, is commonly found in numerous mushroom species, particularly those belonging to the genus Psilocybe, and has a long history of ritual and recreational use. The ritual use of Psilocybe mushrooms dates back thousands of years, with indigenous populations in Mexico and other regions incorporating them into sacred ceremonies. In recent decades, there has been a resurgence of scientific interest in classic psychedelics, such as lysergic acid diethylamide (LSD), psilocybin, 2,5-dimethoxy-4-iodoamphetamine (DOI), N,N-dimethyltryptamine (DMT), and 3,4-methylenedioxymethamphetamine (MDMA), particularly for their potential therapeutic applications. Employing psychedelics such as LSD and psilocybin for treating pain dates back to the 1960s, when their potential therapeutic usefulness in conditions such as cancer-related and phantom limb pain (PLP) was proposed. However, they did not attract much attention due to the complexity of psychedelic experiences, which makes them challenging to study using traditional scientific methods. Recent studies have reignited the field by suggesting that psychedelics may be therapeutically beneficial for managing refractory migraines and cluster headaches, with some recent reviews speculating on the potential mechanisms and applications of these substances in chronic pain management [1].
Mechanism of chronic pain
Chronic pain is a multifaceted phenomenon influenced by various biological, psychological, and social factors. Understanding the interplay between these mechanisms is essential to developing effective strategies for preventing, managing, and treating chronic pain.
Injuries or illnesses can harm the peripheral tissues. The inflammatory mediators released owing to tissue damage increase the sensitivity of peripheral nociceptors. Peripheral sensitization leads to hyperalgesia—an increased perception of pain. Prolonged nociceptive input into the peripheral tissues can lead to central sensitization, which amplifies pain signals within the central nervous system (CNS). Activation of immune cells in the CNS further exacerbates pain sensitivity [2]. Peripheral nerve injuries can lead to neuropathic pain, which is characterized by shooting or burning sensations and numbness. Neuropathic pathophysiology involves abnormal signaling in the damaged nerves and maladaptive changes in the CNS. Chronic pain can prompt structural changes within the nervous system, including reorganizing synaptic connections, changes in brain functional connections, and alterations in neuronal morphology [3].
Neuroplasticity refers to the brain’s ability to modify its structure and function in response to various stimuli, including chronic pain. Neuroplastic changes may include altered gray matter density and volume in regions associated with pain perception and modulation, increased synaptic strength and connectivity in pain-processing pathways, changes in neurotransmitter levels and receptor densities, and reorganization of cortical connections, leading to the hyperactivity of pain-related regions [4].
Psychological factors, such as depression and anxiety, can influence the perception of pain and contribute to the shift from acute to chronic pain. Furthermore, chronic pain can induce emotional distress, resulting in a cycle that worsens the pain symptoms [5].
Pharmacological management of chronic pain typically entails a combination of medications designed to alleviate discomfort and enhance the overall quality of life. NSAIDs and acetaminophen are first-line treatments. Opioids are commonly used to treat severe pain that is unresponsive to other therapies. Tricyclic antidepressants (amitriptyline and nortriptyline) and selective serotonin and norepinephrine reuptake inhibitors (e.g., duloxetine) can alleviate the emotional component of pain in some cases [6–8]. Anticonvulsant medications such as pregabalin and gabapentin may manage neuropathic pain by stabilizing irregular electrical activity in the CNS and modulation of neurotransmitter release [9].
Despite the range of pharmacological treatments available, many patients with chronic pain experience insufficient relief or endure the side effects and risks associated with current therapies. NSAIDs and acetaminophen alone are rarely effective in managing chronic pain. Opioids can cause significant side effects and carry the risks of addiction and overdose. Chronic pain often results from complex interactions involving changes in the nervous system, neuroinflammation, and psychological factors. Effective pain management requires therapies that target these mechanisms. The use of psychedelics in the treatment of chronic pain is an area of growing interest and research. Several mechanisms have been proposed to explain how psychedelics might alleviate chronic pain, including adaptive neuroplastic changes, modulation of serotonin signaling, shifts in perception, alterations in mood and cognition, and anti-inflammatory effects. In the subsequent sections, we will present the current understanding of the aforementioned mechanisms.
Drug metabolism, elimination, and pharmacokinetics
Psilocybin
Psilocybin is a psychedelic compound found in certain mushroom species. It lacks pharmacological activity but undergoes metabolism to produce psilocin, the primary active metabolite responsible for the hallucinogenic effects experienced by users [10]. Interestingly, after the oral administration of escalating doses of psilocybin, no trace of the parent compound is detected in plasma or urine samples. Therefore, investigations into the drug’s kinetics have primarily focused on psilocin, which undergoes extensive metabolism in the liver, mainly through demethylation and oxidation by enzymes such as monoamine oxidase and aldehyde dehydrogenase [11], and further metabolism in the small intestine via glucuronidation.
Psilocin is widely distributed in body tissues, indicated by its volume of distribution exceeding total body water. In a study with three human subjects, the bioavailability of orally administered psilocin was 50–70% [12], similar to that in rodents [13]. Psilocin appears in the bloodstream 20–30 minutes after oral administration, peaking within 2–3 hours. However, the conversion of psilocybin to psilocin shows significant variability, as seen in the varied Tmax values reported in different studies [14]. An escalating dose study revealed that maximum psilocin concentrations were dose-dependent, indicating linear pharmacokinetics within the 0.3–0.6 mg/kg dosage range [15].
Psilocin metabolites are primarily excreted via the kidneys, with psilocin-O-glucuronide as the main urinary metabolite, and only a small amount of psilocin is excreted unchanged [16]. The elimination kinetics of psilocin vary among individuals, but a mean terminal elimination half-life of approximately 3 hours was observed across different oral doses of psilocybin. This consistent half-life suggests a dose-independent metabolism of psilocin [15].
LSD
LSD is a potent psychedelic substance that can be taken orally, intravenously, smoked, or snorted. When taken orally, it is quickly absorbed in the gastrointestinal tract with a bioavailability of about 70–72% [17]. Its effects typically start within an hour and can last 6–12 hours, influenced by the pH of the ingested food [18]. In the liver, LSD is metabolized into inactive metabolites, with the primary one being 2-Oxo-3-hydroxy-LSD (Oxo-HO-LSD), which remains in the urine longer than LSD itself [19]. Minor metabolites, including Oxo-HO-LSD and N-dimethyl-LSD (Nor-LSD), are present in blood plasma at very low concentrations. Nor-LSD has a half-life of about 10 hours longer than LSD [20].
Studies in animals show that LSD is efficiently absorbed and quickly distributed from plasma to tissues, accumulating in the liver, kidneys, spleen, brain, muscle, and fat [21]. A 2 μg/kg intravenous dose in humans results in peak plasma concentrations of 6–7 ng/mL within 30 minutes [22]. Lower doses show similar dose-dependent changes in plasma concentrations. LSD’s elimination half-life ranges from 3–4 hours for doses between 5–20 μg [22, 23]. These findings indicate that LSD, whether taken intravenously or orally, is rapidly absorbed and distributed, with peak plasma concentrations occurring shortly after administration. The effects and duration are dose-dependent, with higher doses leading to longer-lasting effects. However, elimination kinetics remain consistent across different doses [23].
MDMA
MDMA, commonly known as ecstasy, is a widely abused psychostimulant with effects similar to those of amphetamines. MDMA also has unique entactogen properties, promoting closeness, interpersonal relationships, and empathy. It affects serotonin, dopamine, and norepinephrine levels in the brain, leading to euphoria, increased sociability, and perceptual changes. It can also exert somatic symptoms such as increased heart rate and blood pressure.
MDMA is metabolized in the liver in two pathways: O-demethylenation and N-dealkylation. This generates metabolites found mainly in plasma and urine, such as 3,4-methylenedioxyamphetamine (MDA) and 4-hydroxy-3-methoxymethamphetamine (HMMA). MDA is a minor metabolite, making up about 8–9% of MDMA’s concentration, while HMMA is the main metabolite, especially at lower MDMA doses [24]. Although MDMA metabolites can be active, they are generally less potent than MDMA itself. However, MDA can still have psychoactive effects, albeit to a lesser extent than those of MDMA.
MDMA binds to plasma proteins at about 34% and MDA at about 40%. The volume of distribution is 453 liters, and the elimination half-life of MDMA is about 8–9 hours, indicating faster clearance compared to methamphetamine or amphetamine. The nonlinear pharmacokinetics of MDMA are likely due to enzyme-metabolite complex formation with CYP2D6 [25].
MDMA is excreted in the urine, with minor excretion pathways involving feces and sweat. Urine is the main route for eliminating MDMA and its metabolites [26].
Mescaline
Mescaline, also known as 3,4,5-trimethoxyphenethylamine, is a naturally occurring psychedelic found in cacti like peyote (Lophophora williamsii) and San Pedro (Echinopsis pachanoi). It is a phenethylamine with hallucinogenic effects similar to LSD and psilocybin [27].
Mescaline affects serotonin levels in the brain, influencing its release and reuptake, with minor effects on dopamine [28]. However, compared to LSD, it has a lower binding affinity for the 5-HT2A receptor, making it the least potent psychedelic among various tryptamines, including psilocin and DMT [29]. Mescaline is not addictive and shows cross-tolerance with other serotonergic drugs, meaning tolerance to one can reduce the effects of the other [30].
Mescaline is rapidly absorbed from the gastrointestinal tract and distributed to the kidneys and liver, delaying its entry into the bloodstream and extending its half-life to about 6 hours in humans [31]. The peak effects of mescaline occur about 2 hours after ingestion and last 10–12 hours [32]. Mescaline is primarily metabolized in the liver through oxidative deamination, converting it into inactive compounds like 3,4,5-trimethoxyphenylacetic acid and 3,4,5-trimethoxyphenylethanol, which are excreted in the urine [33]. Around 81% of the ingested dose is eliminated unchanged within the first hour, with other metabolites like N-acetyl mescaline also being excreted in the urine [34].
Finally, the long-term effects of psychedelics on psychological and cognitive health are not well understood despite their increasing use. Table 1 summarizes the pharmacokinetics and pharmacodynamics of the aforementioned psychedelic drugs. There is growing interest in their potential therapeutic applications in psychiatry and pain management, which could lead to better treatment options and a deeper understanding of their effects.
Pharmacology of the psychedelics
| Substance | Mechanism of action | Pharmacokinetics |
|---|---|---|
| Psilocybin | Metabolized in the body to psilocin, which primarily binds to serotonin receptors (5-HT2A) in the brain | Dose-independentBioavailability: 50–70% (oral)Onset of action: 20–30 minutesTime to peak plasma concentration: 2–3 hoursHalf-life: 1.5–3 hours (psilocin)Duration of action: 2–6 hours |
| LSD | Binds primarily to serotonin receptors (5-HT2A) in the brain | Dose-dependentBioavailability: 70–72% (oral)Onset of action: 60 minutesTime to peak plasma concentration: 30 minutesHalf-life: 3–4 hoursDuration of action: 6–12 hours |
| MDMA | Increases serotonin, dopamine, and norepinephrine levels in the brain by inhibiting their reuptake | Dose-dependentOnset of action: 30–60 minutesTime to peak plasma concentration: 2 hoursHalf-life: 8–9 hoursDuration of action: 6–12 hours |
| Mescaline | Binds to serotonin receptors (5-HT2A) and dopamine receptors | Dose-dependentOnset of action: 30 minutesTime to peak plasma concentration: 2 hoursHalf-life: 6 hoursDuration of action: 10–12 hours |
LSD: lysergic acid diethylamide; MDMA: 3,4-methylenedioxymethamphetamine
Potential mechanisms and connectivity
The neuropharmacological foundation for the potential effectiveness of classical psychedelics such as LSD, psilocybin, MDMA, and mescaline for chronic pain remains unclear and lacks a definitive consensus. Nevertheless, notable observations are prompting further research, particularly to elucidate the mechanisms through which psychedelics may reduce persistent pain and the distress associated with it.
Serotonergic pathway and pain-related emotional distress
Psychedelics mainly work by activating the 5-HT2A receptor. Studies show that blocking this receptor with substances like ketanserin can prevent the psychedelic effects of drugs like LSD. Imaging experiments have also linked the effects of psilocybin to the activation of the 5-HT2A receptors, which are abundant on the glutamatergic “excitatory” pyramidal cells in layer V of the cortex, with a lesser expression on the “inhibitory” GABAergic interneurons [35].
It is widely acknowledged that 5-HT receptors play a pivotal role in pain perception. These receptors send signals to the dorsal horn in the spinal cord, significantly influencing pain perception. The 5-HT pathway can either inhibit or stimulate pain, depending on whether the pain is acute or chronic. The 5-HT pathway often exhibits a pain-relieving effect in chronic pain, while peripheral 5-HT2A receptors can promote inflammatory pain. Studies suggest that LSD can inhibit pain by acting on 5-HT1A receptors in the dorsal raphe, a brain area intricately involved in pain inhibition [36].
It has been established that psychedelic drugs induce redistribution of 5-HT2A receptors both in vivo and in vitro from the cell surface to intracellular compartments [37], resulting in a reduction in the total number of cell surface 5-HT2A receptor-binding sites in the pyramidal neurons. This restructuring consequently mitigates pain sensation, particularly inflammatory pain, as 5-HT2A receptors typically depolarize dorsal root ganglia (DRG) neurons [38]. Nevertheless, terminally ill patients with chronic pain often experience emotional, existential, and spiritual suffering. The distress can lead to a profound ‘sense of doom’, where the patient feels like their life lacks meaning. Psychedelics can ease these sensations through mystical-like subjective effects, potentially alleviating pathological self-fixation, such as rumination and catastrophizing, which are often seen in patients with chronic pain [39].
Dopaminergic pathway and mesolimbic system
The dopaminergic pathways in the brain contribute to pain perception, particularly through the mesolimbic system. This system includes dopaminergic projections from the ventral tegmental area (VTA) in the midbrain to the nucleus accumbens in the limbic system and is associated with motivated behavior and drug reward [40]. Psychedelics can indirectly enhance dopamine transmission by acting on 5-HT2A receptors. For instance, the 5-HT2A agonist DOI increases the activity of dopaminergic neurons in the VTA, resulting in increased dopamine release in the VTA and its projection areas. This effect is facilitated by the activation of 5-HT2A receptors in the prefrontal cortex, which provides excitatory input to the VTA [41]. While mesolimbic dopamine activation may contribute to the pain relief seen with psychedelics, some studies show that drugs like phencyclidine act on NMDA receptors in the nucleus accumbens independently of dopamine [42]. Regardless of the mechanism of action and connections within the reward circuitry linking the frontal cortex and nucleus accumbens, these structures are associated with the rewarding effects of psychomotor stimulants.
In summary, neuropharmacological mechanisms underlying the potential pain-relieving effects of classic psychedelics such as psilocybin and LSD are not fully understood. However, they primarily activate 5-HT2A receptors, which may affect pain perception and emotional well-being. Psychedelics also enhance dopamine transmission, which could contribute to their pain-relieving effects. Based on these findings, more research is needed to develop new treatments for chronic pain.
Connectivity and neuroplasticity
Classic psychedelics like LSD and psilocybin can lead to lasting improvements in conditions such as depression, anxiety, and addiction. One theory is that psychedelics increase neuroplasticity, resulting in long-lasting brain changes [43, 44]. Animal studies show that LSD, psilocybin, DMT, and DOI promote genes related to synaptic plasticity and increase synaptic and dendritic growth [45–47]. However, their impact on neurogenesis varies, with some substances showing no effect and others, like DMT, increasing neurogenesis. Human studies using peripheral brain-derived neurotrophic factor (BDNF) as a neuroplasticity marker have shown mixed results [48].
The 5-HT2A receptor is key to psychedelic-enhanced neuroplasticity. When psychedelics stimulate these receptors, they increase glutamate levels and activate AMPA receptors. This starts a feedback loop involving BDNF secretion and mTOR activation, promoting dendritic growth. Psychedelics enhance neuroplasticity, especially in areas with high 5-HT2A receptor expression, like the neocortex [49]. Other cortical regions also show enhanced neuroplasticity due to 5-HT2A receptor effects. The hippocampus shows more modest effects, likely because of more 5-HT1A receptors.
The dopaminergic mesolimbic pathway is less affected by psychedelics because of lower 5-HT2A receptor expression. However, psychedelics might relieve chronic pain by modulating inhibitory neurons in the prefrontal cortex that project to the mesolimbic pathway. Neuroimaging studies show that psychedelics change large-scale brain networks, leading to changes in brain function that could affect long-term outcomes. For example, fMRI studies reveal that psilocybin decreases cerebral blood flow in certain brain regions and reduces connectivity between cortical areas, similar to patterns seen in general anesthesia (Figure 1) [50].
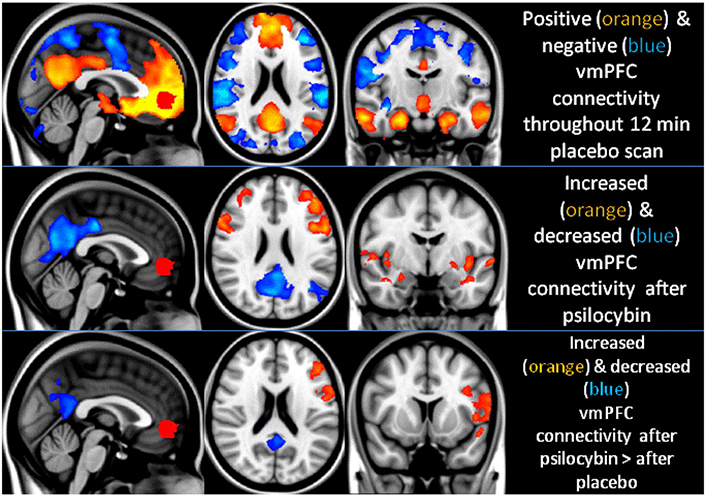
Psilocybin modifies the functional connectivity of the ventromedial prefrontal cortex (vmPFC). Positive and negative couplings with vmPFC activity are visually represented in orange and blue, respectively. After psilocybin infusion, connectivity markedly increases (orange) and decreases (blue) compared to a placebo. These changes are particularly notable in the posterior cingulate cortex (PCC) and left lateral parietal region, indicating a significant reduction in positive coupling rather than the emergence of new negative coupling
Note. Reprinted with permission from “Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin” by Carhart-Harris RL, Erritzoe D, Williams T, Stone JM, Reed LJ, Colasanti A, et al. Proc Natl Acad Sci U S A. 2012;109:2138–43 (https://doi.org/10.1073/pnas.1119598109) © National Academy of Sciences 2024.
The transition from acute to chronic pain involves changes in brain connectivity. Psychedelics can disrupt established connectivity patterns and enhance global integration, suggesting a potential mechanism for their effects on chronic pain [51, 52]. Understanding how psychedelics enhance neuroplasticity and alter brain connectivity could lead to new treatments, especially for chronic pain, where current therapies often fall short.
Psychedelics and long-term effectiveness
The timing and duration of increased neuroplasticity after taking psychedelics vary across studies and species. In rodents, neuroplasticity-related changes can start within an hour, with cellular changes seen around six hours later. In humans, increased levels of peripheral BDNF have been observed as soon as four hours after taking LSD. These neuroplastic changes can last for days to weeks, with some studies showing elevated BDNF levels and altered brain function up to a month after treatment. Considering the long-term management of chronic pain, it is important to note that after the acute psychedelic experience, patients often report clearer thinking, greater emotional openness, and reduced anxiety and depression. These benefits can last weeks or months, aligning with findings from marker studies. Different markers of neuroplasticity may have different time courses, so more research is needed to fully understand the timing of these changes in humans [53]. This understanding could help optimize the timing of psychotherapeutic interventions.
Clinical applications in chronic pain
Phantom limb pain
PLP is characterized by a sensation of pain in a limb that was amputated and affects approximately 78% of amputees. While the mechanisms underlying PLP are unclear, cortical remapping and reorganization of somatosensory maps following limb loss are believed to cause PLP. The damage caused by amputations disrupts normal afferent and efferent signals in the peripheral nerves, and the formation of neuromas exacerbates this effect by increasing neuronal excitability [54].
Currently, the management of PLP involves symptomatic control and mitigating neuropathic pain with medications such as gabapentin, pregabalin, and antiepileptics. However, the lack of consensus on treatment has led to exploring alternative therapies.
Kuromaru et al. conducted the first study on the potential of psychedelics in alleviating PLP. In this study, 50 µg of LSD were administered to eight patients with persistent PLP. Among these eight patients, seven reported significant and sustained pain relief. Although minimal mood changes were observed as a side effect, no severe psychological disorders were observed. This study hypothesized that LSD relieved PLP by inducing changes in patients’ psychological framework [55].
Fanciullacci et al. implemented a similar study protocol involving seven patients with PLP. Using a similar protocol, patients received escalating doses of LSD over several weeks, resulting in a notable 71% reduction in pain and 50% decrease in analgesic consumption. Transient psychiatric reactions have also been reported as side effects. The authors concluded that central potentiation of 5-HT activity occurs via pathways involved in central pain regulation [56].
Most recently, Ramachandran et al. integrated psilocybin with mirror visual feedback (MVF). MVF is known for its efficacy in creating the illusion of a complete limb using a mirror. A combination of psilocybin and MVF enhanced pain relief and duration. The sustained reduction in pain was attributed to increased functional connectivity and activation of 5-HT2A receptors [57].
Fibromyalgia
Fibromyalgia commonly presents with widespread musculoskeletal pain and is often accompanied by fatigue, sleep disturbances, and cognitive difficulties. This chronic pain condition affects an estimated 2–8% of the population and predominantly targets women. While the etiology of fibromyalgia remains unclear, current research suggests that fibromyalgia occurs because of abnormalities in central pain processing, resulting in hyperalgesia and allodynia. This may be associated with increased pronociceptive neurotransmitters such as glutamate and substance P. Evidence supports a multifaceted approach to the management of fibromyalgia, including medications such as tricyclic acids and dual reuptake inhibitors, cardiovascular exercise, and cognitive-behavioral therapy (CBT) [58].
However, there is a growing interest in exploring unconventional therapeutic approaches. Glynos et al. investigated the potential of psychedelics through a cross-sectional, anonymous, online survey including 354 North American participants with fibromyalgia. 29.9% of participants reported previous use of psychedelics, and among the 12 patients who used psychedelics with the intent to alleviate chronic pain, 11 reported symptom improvement. Collective feedback from the participants underscores the potential role of psychedelics in the management of fibromyalgia pain, prompting further investigation into their therapeutic utility [59]. However, it is important to note that online survey studies have limitations, such as self-reporting bias, lack of control over participant selection, and potential inaccuracies in data collection. Moreover, the literature in this area is scarce, highlighting the need to conduct reliable, well-designed studies to better understand the efficacy and safety of psychedelics in treating fibromyalgia.
Cluster headache
Cluster headache, which affects approximately 0.1% of the global population, manifest as excruciating unilateral pain in the orbital, supraorbital, or temporal regions, often accompanied by ipsilateral autonomic symptoms. This condition is characterized by a circadian rhythmicity of intense but brief attacks, often recurring in bouts interspersed with periods of remission. The pathophysiology suggests the activation of the trigeminovascular system, leading to neurogenic inflammation and trigeminal autonomic reflex excitation [60]. Due to the refractory nature of conventional treatments such as oxygen therapy, triptans, and calcium channel blockers, the exploration of adjunctive therapeutic modalities was first conducted by Sicuteri [61], who examined the effectiveness of LSD in relieving cluster headache attacks, sparking an interest in subsequent research.
Subsequent investigations of the potential of psychedelics in cluster headache management have provided further insights. Sewell et al. conducted interviews with 53 patients with cluster headaches who used psilocybin or LSD to manage their conditions. While 22 of the 26 psilocybin users reported effective cluster headache attack abortion, 25 of the 48 psilocybin users, and 7 of the 8 LSD users reported cluster period termination. Additionally, many reported a prolonged remission period [62].
Similarly, a survey by Schindler et al. in 2015 recruited 496 patients with cluster headaches to characterize the effects of conventional and alternative medications. These responses indicate that psilocybin, LSD, and related psychedelic compounds exhibited comparable or superior efficacy to conventional treatments in aborting attacks and inducing remission [63].
In a study exploring treatment modalities of cluster headaches, Di Lorenzo et al. surveyed 54 respondents with chronic or drug-resistant cluster headaches. All patients reported dissatisfaction with conventional treatments and turned to illicit substances for therapeutic purposes. 85.7% of participants perceived psychedelic agents as safe or even safer than conventional medications [64]. Qualitative analyses by Andersson et al. supported these findings, emphasizing the efficacy of psychedelics, particularly psilocybin, and LSD, in both prophylactic and acute treatment of cluster headaches and migraines, with no severe adverse events reported [65].
Schindler et al. most recently conducted an exploratory clinical trial to investigate the efficacy of psilocybin in suppressing cluster headaches. Thirty participants were randomized to receive psilocybin or placebo at three doses, each spaced approximately five days apart. Results of the study showed that psilocybin administration reduced cluster attack frequency with no serious adverse effects, further emphasizing its potential as a therapeutic agent for cluster headache management [66].
Cancer pain
To identify an analgesic offering prolonged relief with minimal side effects, Kast and Collins investigated the use of LSD-25 in 50 patients with cancer pain. This double-blind clinical trial administered 100 μg of LSD to patients, comparing its efficacy to hydromorphone and meperidine. Within three hours of drug administration, LSD-25 exhibited superior effectiveness over both meperidine and dihydromorphinone at a statistically significant level (P < 0.001). Notably, LSD demonstrated not only more potent analgesic action but also provided more enduring pain relief compared with conventional analgesics [67].
Following these pioneering efforts, Pahnke et al. [68] and Grof et al. [69] conducted similar studies employing LSD-assisted psychotherapy in patients with terminal cancer. The study of Grof et al. [69] included 60 patients with cancer, 44 patients received 200–500 μg of LSD. Although not statistically significant, a reduction in the use of narcotic medication was observed. Moreover, 29% of the patients experienced dramatic improvements in emotional and physical distress, whereas 41.9% exhibited moderate improvements [68, 69].
More recently, research has emerged evaluating psilocybin as a potential treatment for patients with cancer pain, particularly for alleviating the associated anxiety and depression. Initial pilot studies led by Grob et al. [69] paved the way for further investigations conducted by Griffiths et al. [70] and Ross et al. [71]. These studies have consistently demonstrated reductions in measures of anxiety and depression, decreased fear of death, and improvements in overall well-being, quality of life, and spirituality among patients with pain cancer [70–72].
In 2022, Maia et al. [73] conducted a systematic review of 20 studies exploring the effects of LSD, psilocybin, and N,N-dipropyltryptamine in cancer patients. The analysis concluded that psychedelic-assisted therapies significantly improved symptom control, especially the psychological and spiritual symptoms associated with severe illnesses [73].
Despite their promising therapeutic potential, adverse effects of psychedelic-assisted therapies have been noted, ranging from physical effects, such as visual disturbances, nausea, and changes in vital signs, to psychological manifestations, including fear, panic, hallucinations, and emotional distress. These findings underscore the need for further research to optimize the therapeutic benefits while mitigating adverse effects in cancer patients undergoing psychedelic-assisted therapies.
Regulatory considerations
Psilocybin and other psychoactive substances are classified as Schedule I drugs under the Controlled Substances Act of 1970 [36]. Before this legislation, psilocybin was sold in the USA under the trade name indocybin and was frequently used as an adjunct to psychotherapy [74]. Despite its efficacy, this backlash led to a ban on the marketing and possession of psychedelic substances in 1965, and indocybin was discontinued in 1966 [75].
The potential removal of psilocybin from Schedule I for a demonstrable therapeutic effect would require an assessment of potential abuse potential by the FDA. Although the risk of overdose is relatively low, owing to low physiological toxicity, the psychological alterations that occur subsequent to ingestion may cause a user to harm themselves or others, a sensation known to be disturbing to participants [76]. Increased dosages of psilocybin are known to increase psychological distress and the potential risk of violence or seeking medical assistance for enduring symptoms after usage has terminated [76]. Nonetheless, psilocybin is safe when used at appropriate dosages and under medical supervision [70].
LSD was first sold as a psychiatric drug by Sandoz Laboratories in 1947 and marketed as a treatment for schizophrenia, criminal behavior, and alcoholism, among other conditions [77]. In the 1950s, the US Central Intelligence Agency studied LSD through its covert program, Project MK-ULTRA, in which experiments were conducted without the participants’ knowledge to study the effects of psychoactive substances [78]. Prominent figures such as Aldous Huxley and Allen Ginsberg advocated the use of LSD in the 1960s and cemented its place in the counterculture movement of the decade [79, 80]. In 1968, however, the possession of LSD in the USA was illegal, although, in November 2020, Oregon became the first US state to decriminalize the possession of small amounts of LSD via Ballot Measure 110 [81].
MDMA was first patented in 1912 by Merck. However, its use, recreationally and in research, remained sporadic until the late 1960s [82]. It has gained favor among psychiatrists for treating depression and substance use disorders, among other conditions [83]. The recreational market developed in the late 1970s and the 1980s, gaining prominence in nightclubs such as Studio 54 and Paradise Garage [84]. In 1981, it was rebranded as “ecstasy” by Michael Clegg, a prominent distributor of the drug, as its use continued to spread [84].
Widespread recreational use led the DEA to classify MDMA under Schedule I in May 1985 [85]. Although psychiatrists have made efforts to vacate this regulation to maintain its use in therapeutic modalities, Schedule I remains. In 2017, however, the FDA granted MDMA a breakthrough therapy designation to enable its use in PTSD psychotherapy [86].
Mescaline became illegal in 1970 following the passage of the Comprehensive Drug Abuse Prevention and Control Act [87]. The 1971 Convention on Psychotropic Substances banned its use internationally [88]. Some religious groups are excluded from such prohibitions, including the Native American Church, through the passage of the American Indian Religious Freedom Act of 1978, although these laws have been challenged in some states [89].
The future of psychoactive substance regulation, especially for medical use, remains in flux. Although these drugs remain banned at the federal level, efforts by individual states and municipalities to reduce their use have persisted [90]. Systematic reviews have demonstrated the great benefits of psychedelic use for conditions such as PTSD, major depression, addiction, anxiety and renewed efforts for rescheduling or decriminalization. In 2023, twenty states introduced psychedelic-related legislation ranging from enabling some research to recreational use by the public [91].
There is evidence that current regulatory, ethical, and legal frameworks are rapidly evolving to incorporate the use of psychoactive substances in healthcare. For example, in 2018, Compass Pathways, a biosciences company that seeks evidence-based pathways for the treatment of mental health disorders, received a breakthrough therapy designation from the FDA for psilocybin therapy use in treatment-resistant depression [92]. The breakthrough therapy designation is generally reserved for therapies that have the potential to be substantially better than currently accepted modalities. In January 2024, the Department of Veterans Affairs issued a request for proposals from affiliated researchers for investigations using psychedelics for PTSD and depression [93]. Approval by regulatory agencies and new sources of funding are likely responses to pressure from such influential groups as the American Psychiatric Association (APA), which released a position statement regarding the use of psychedelics for mental health in 2022 [94]. Nonetheless, despite all of these positive developments, there have still been setbacks for the use of psychedelics for medicinal purposes. In June of 2024, Lykos Therapeutics failed to gain FDA advisory board approval for the use of MDMA for PTSD. Upon further review, however, this regulatory challenge was likely more related to poor clinical design and execution than the merits of the proposed therapeutics [95]. Moving forward, investment and buy-in from professional societies such as the APA and private corporations such as Compass Pathways will almost certainly lead to success in the face of these challenges. These gains will continue to erode roadblocks for the use of psychedelics in healthcare due to their therapeutic and profit potential.
Conclusions
In conclusion, this review provides a relatively comprehensive insight into the efficacy, mechanisms, and adverse effects of psychedelics in populations with various subtypes of chronic pain. Research suggests that psychedelics can induce an altered state of consciousness that may lead to profound shifts in perception, cognition, and emotional processing. These altered states may allow patients to perceive their pain differently, potentially reducing its intensity or emotional impact. While the potential benefits of psychedelics for chronic pain management are promising, it is important to note that research in this area is still in its early stages. Clinical trials are ongoing to better understand the safety, efficacy, and optimal dosing regimens of psychedelic drugs for pain management. However, their use carries risks, including psychological distress, adverse reactions, and legal implications. Overall, while psychedelics show promise as a novel approach for chronic pain management, more research is needed to fully understand their mechanisms of action and determine their long-term safety and effectiveness.
Abbreviations
| BDNF: | brain-derived neurotrophic factor |
| CNS: | central nervous system |
| DOI: | 2,5-dimethoxy-4-iodoamphetamine |
| DMT: | N,N-dimethyltryptamine |
| LSD: | lysergic acid diethylamide |
| MDA: | 3,4-methylenedioxyamphetamine |
| MDMA: | 3,4-methylenedioxymethamphetamine |
| MVF: | mirror visual feedback |
| PLP: | phantom limb pain |
| VTA: | ventral tegmental area |
Declarations
Author contributions
BY: Data curation, Methodology, Project administration, Formal analysis, Writing—original draft, Writing—review & editing. SM: Data curation, Validation, Writing—original draft. GT: Methodology, Validation, Writing—original draft, Writing—review & editing. AB: Conceptualization, Methodology, Supervision, Writing—original draft, Writing—review & editing.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2024.