Affiliation:
1CEA, List, Université Paris-Saclay, Palaiseau 91120, France
2Institut de Neurosciences des Systèmes, Aix-Marseille University, INSERM, 13007 Marseille, France
Email: swatibanerjee@ieee.org
ORCID: https://orcid.org/0000-0003-1214-8144
Affiliation:
2Institut de Neurosciences des Systèmes, Aix-Marseille University, INSERM, 13007 Marseille, France
ORCID: https://orcid.org/0000-0002-8251-8860
Explor Neurosci. 2024;3:478–492 DOI: https://doi.org/10.37349/en.2024.00060
Received: June 24, 2024 Accepted: July 30, 2024 Published: September 29, 2024
Academic Editor: Jinwei Zhang, University of Exeter Medical School, UK
The article belongs to the special issue Epilepsy
Epilepsy, a neurological disorder characterized by recurrent seizures, presents a complex interplay of cellular and molecular mechanisms. The symptoms manifest themselves at various scales, from ion channels to brain regions to behavior in humans. Various screening, treatment, and preventive measures use this knowledge to tackle the disorder effectively. This article aims to summarize the current state of the art in epileptic markers from ion channels, astrocytes, and synaptic imbalance to whole brain Network Dynamics. Recent research has shed light on the critical involvement of astrocytes, the multifunctional glial cells, in the pathogenesis and modulation of epileptic seizures in humans. Astrocytes, once considered as mere supportive cells, are now recognized as active participants in the regulation of neuronal excitability, synaptic transmission, and brain homeostasis. Ion channel imbalance is one of the widely studied areas in the context of epilepsy and is partially addressed in the abstract. Recent advances in computational neuroscience have led to the development of whole brain network models, providing valuable tools for studying the complex dynamics of epileptic seizures. These models integrate diverse biological factors, including neuronal connectivity, synaptic dynamics, and cellular properties, to simulate the spatiotemporal patterns of epileptic activity across brain regions. Through computational simulations and analysis, whole brain network models offer insights into seizure initiation, propagation, and termination mechanisms, shedding light on the dynamic interactions between epileptic foci and distributed brain networks. Moreover, these models facilitate the exploration of network-based biomarkers for seizure prediction and intervention optimization. Challenges and limitations, such as model complexity and validation against experimental data, are also discussed. Despite these challenges, whole brain network models represent a promising approach for advancing our understanding of epilepsy and identifying novel therapeutic strategies. Future research efforts should focus on refining model fidelity, incorporating multimodal data, and translating computational findings into clinically relevant applications, ultimately improving the management and treatment of epilepsy patients.
Epilepsy, a condition affecting millions worldwide, is characterized by the occurrence of recurrent seizures. According to the World Health Organisation (WHO) [1]. The estimated proportion of the general population with active epilepsy (i.e., continuing seizures or with the need for prevention and treatment) at a given time is between 4 and 10 per 1,000 people. Globally, an estimated 5 million people are diagnosed with epilepsy each year. In high income countries, there are estimated to be 49 per 100,000 people diagnosed with epilepsy each year. In low and middle income countries, this figure can be as high as 139 per 100,000. This is usually due to the increased risk of other endemic conditions. Close to 80% of people with epilepsy live in low and middle income countries and do not receive proper care and sometimes not even proper diagnosis.
Globally, an estimated 5 million people are diagnosed with epilepsy each year. In high-income countries, it is estimated that 49 per 100,000 people are diagnosed with epilepsy each year. In low and middle income countries, this figure can be as high as 139 per 100,000. This is likely due to the increased risk of endemic conditions such as malaria or neurocysticercosis, the higher incidence of road traffic injuries, birth related injuries, variations in medical infrastructure, and the availability of preventive health programs and accessible care. Close to 80% of people with epilepsy live in low and middle-income countries as described by WHO [1].
The emergence of seizures results from abnormal electrical activity in the brain, and unraveling their physiological basis is crucial for comprehending the pathophysiology of epilepsy [2]. Seizure episodes are a result of excessive electrical discharges in a group of brain cells. Different parts of the brain can be the site of such discharges. Seizures can vary from the briefest lapses of attention or muscle jerks to severe and prolonged convulsions. Seizures can also vary in frequency, from less than one per year to several per day.
In this manuscript, we look at epilepsy at various scales. Prompt and efficient treatment often requires prior knowledge or predictability, of when and where seizures are likely to occur. Developing prediction strategies is extremely challenging due to the patient specific causes of seizures, and the difficulty in obtaining data from longitudinal studies.
The fundamental mechanism underlying neuronal excitability revolves around the concept of action potentials. A state of heightened excitability can arise from various factors, including increased excitatory synaptic transmission, reduced inhibitory neurotransmission, alterations in voltage gated ion channels, or changes in intra-or extracellular ion concentrations favoring membrane depolarization. Additionally, hyper-excitability can occur when multiple subthreshold excitatory stimuli synchronize, allowing their temporal summation in postsynaptic neurons [3].
Action potentials occur due to membrane depolarization in neurons, propagating along the axon and triggering neurotransmitter release at axon terminals. These action potentials manifest as all-or-none events, influenced by local changes in membrane potential resulting from net positive inward ion flows. Membrane potential is further modulated by the activation of ligand-gated channels, affected by neurotransmitter binding, and voltage-gated channels, influenced by alterations in transmembrane potential, and changes in intracellular ion compartmentalization [3].
Ion channels are integral membrane proteins that are crucial for regulating the flow of ions such as sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl–) across neuronal membranes. These channels play a pivotal role in maintaining the delicate balance of ion concentrations inside and outside neurons, which is essential for proper neuronal function [3]. They are classified into two main types: voltage-gated and ligand-gated channels.
Voltage-gated ion channels respond to changes in membrane voltage. When the membrane potential reaches a certain threshold, voltage-gated ion channels open or close, allowing specific ions to flow in or out of the cell. This process is vital for generating and propagating action potentials. Sodium channels, potassium channels, and calcium channels are examples of voltage-gated ion channels.
Ligand-gated ion channels are activated by the binding of specific neurotransmitters or ligands. When neurotransmitters bind to these receptors, the ion channels open, leading to changes in membrane potential. One example is the N-methyl-D-aspartate (NMDA) receptor, which plays a crucial role in synaptic plasticity and learning and memory [3].
Voltage-gated ion channels open or close in response to changes in membrane potential. When the membrane potential depolarizes (becomes less negative), sodium channels typically open, allowing sodium ions to rush into the cell, leading to depolarization and the initiation of an action potential. Conversely, potassium channels open during repolarization, allowing potassium ions to leave the cell and restore the membrane potential to its resting state.
Ligand-gated ion channels, on the other hand, open when specific neurotransmitters bind to them. For example, when glutamate, the major excitatory neurotransmitter, binds to NMDA receptors, the channel opens, permitting the flow of calcium ions and contributing to synaptic transmission and plasticity [3]. In epilepsy, dysfunction of ion channels represents a critical component of the underlying pathology. Genetic mutations in genes encoding ion channels or associated proteins can disrupt the proper function of these channels, leading to an increased risk of seizures.
One notable example is the SCN1A gene, which encodes the sodium channel Nav1.1. Mutations in SCN1A have been associated with various forms of epilepsy, including Dravet syndrome, a severe and drug-resistant epileptic encephalopathy. These mutations can lead to increased neuronal excitability and abnormal firing patterns due to impaired sodium channel function. As a result, individuals with SCN1A mutations often experience recurrent seizures [4].
Furthermore, mutations in other ion channel genes, such as potassium channels (e.g., KCNQ2) and calcium channels (e.g., CACNA1A), have also been linked to different epilepsy syndromes. These mutations disrupt the normal regulation of ion flow, leading to hyperexcitability and the generation of epileptic seizures [5].
Understanding the intricate role of ion channels and their dysfunction in epilepsy is crucial for the development of targeted therapies and interventions aimed at restoring normal neuronal excitability and mitigating seizure activity [4, 5]. Furthermore, ion channel dysfunction can disrupt the delicate balance between excitatory and inhibitory neurotransmission, leading to increased excitatory signaling and reduced inhibitory control within the neural circuits, which are critical factors contributing to epileptic hyperexcitability.
Neurotransmitters are molecules released by presynaptic nerve terminals at synapses, binding to specific postsynaptic receptors. Ligand binding activates channels, permitting ion movement into or out of cells. Major neurotransmitters in the brain include glutamate, gamma-aminobutyric acid (GABA), acetylcholine (ACh), norepinephrine, dopamine, serotonin, and histamine. Other molecules, like neuropeptides and hormones, play modulatory roles that alter neurotransmission over extended time periods. The primary excitatory neurotransmitter is glutamate, with several subtypes of glutamate receptors. These receptors are found postsynaptically on excitatory principal cells, inhibitory interneurons, and certain glial cell types. Ionotropic subclasses include alpha-amino-2, 3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA), kainate receptors, and NMDA. They permit ion flux upon glutamate activation and are differentiated by cation permeability and sensitivity to pharmacological agents. All ionotropic glutamate receptors allow Na+ and K+ permeability, contributing to membrane depolarization and action potential generation [6]. NMDA receptors include a Ca2+ channel, blocked by Mg2+ ions at rest but unblocked during local membrane depolarization, potentially leading to further depolarization and excitotoxicity in conditions of excessive neuronal activation such as status epilepticus and ischemia. Another major glutamate receptor type is metabotropic, operating through receptor-activated signal transduction with membrane-associated G-proteins. There are at least three subtypes of metabotropic receptors based on agonist potency, signal transduction mechanism, and pre- or post-synaptic localization [6].
The interplay between excitatory and inhibitory neurotransmitters is central to regulating neuronal activity. Imbalances in these neurotransmitter systems, such as decreased GABAergic inhibition, can precipitate epileptic activity [7]. GABA, the principal inhibitory neurotransmitter in the brain, is often compromised in epilepsy.
Astrocytes, a type of glial cell in the central nervous system, play several important roles in regulating ion channels, which are essential for neuronal function.
Astrocytes play a significant role in epilepsy, which is a genre of neurological disorder characterized by recurrent seizures. Their involvement in epilepsy is multi-faceted, contributing to both the development and modulation of seizures. Below are some points related to astrocyte and their association with epilepsy [8–10].
Potassium buffering: astrocytes are crucial for maintaining potassium ion ([K+]) homeostasis in the brain. During seizures, there can be excessive neuronal firing, leading to increased extracellular potassium levels. Astrocytes normally help clear this excess potassium to restore balance. However, in chronic epilepsy, alterations in astrocytic potassium buffering capacity can contribute to hyperexcitability, making it easier for seizures to occur. Glutamate clearance: glutamate is the primary excitatory neurotransmitter in the brain, and its excessive release can lead to seizures. Astrocytes are responsible for clearing glutamate from the synaptic cleft. Dysfunction in glutamate transporters on astrocytes, such as glutamate transporter-1 (GLT-1) excitatory amino acid transporter 2 (EAAT2), can result in prolonged glutamate exposure and excitotoxicity, which can contribute to seizure generation and propagation. Reactive gliosis: in response to repeated seizures or brain injury, astrocytes undergo a process called reactive gliosis. Reactive astrocytes can release pro-inflammatory cytokines, chemokines, and other signaling molecules that promote neuronal hyperexcitability and seizure susceptibility [8–10].
Gliotransmission: astrocytes release gliotransmitters such as ATP, D-serine, and glutamate in response to neuronal activity. These gliotransmitters can modulate synaptic transmission and neuronal excitability. In epilepsy, aberrant gliotransmission can contribute to seizure generation or propagation.
Neurovascular coupling: astrocytes are involved in neurovascular coupling, the process by which local neuronal activity leads to changes in blood flow. Altered neurovascular coupling in epilepsy can affect the delivery of oxygen and nutrients to neurons, potentially exacerbating seizure activity [11, 12].
Scar formation: in cases of severe brain injury or repeated seizures, astrocytes can contribute to the formation of glial scars. While these scars can limit the spread of seizure activity, they can also create a barrier that hinders normal neuronal communication, potentially contributing to epileptogenesis (the process of developing epilepsy).
Ion channel dysfunction: astrocytes express various ion channels that regulate their functions. Dysfunction in these channels, such as inward-rectifying potassium (Kir) channels, can impair astrocytic potassium buffering capacity and contribute to hyperexcitability. Metabolic changes: astrocytes are critical for providing energy substrates to neurons through processes like glycolysis. Dysfunctional astrocytic metabolism, which can occur in epilepsy, may impair their ability to support neurons during periods of increased activity, further promoting seizures [13, 14].
Astrocyte-targeted therapies: given their diverse roles in epilepsy, astrocytes have emerged as potential therapeutic targets. Researchers are exploring ways to modulate astrocytic functions, such as enhancing potassium buffering capacity or improving glutamate clearance, as strategies to reduce seizure frequency and severity. In summary, astrocytes are intimately involved in the pathophysiology of epilepsy. Epilepsy being a network disease studying the role of astrocytes in network modulation gives valuable insight [13, 14]. Their roles in maintaining ion homeostasis, neurotransmitter clearance, and modulating neuronal excitability make them key players in both the generation and modulation of seizures. Understanding the complex interactions between astrocytes and neurons in epilepsy may lead to new therapeutic approaches for this challenging neurological disorder.
This section explains and summarizes, neural excitability and the associated brain regions to be able to understand the abnormality arising in terms of network synchronization or perturbation.
Epileptic seizures frequently entail the synchronized firing of large neuronal populations. This synchronization can cause the propagation of epileptic activity throughout the brain [15]. Factors contributing to this phenomenon may include structural brain lesions or abnormal connectivity.
To elucidate the physiological basis of epilepsy, it is imperative to identify and understand the brain areas typically involved in seizure generation and propagation. Figure 1 shows a meso to macroscale view of temporal lobe epilepsy. The next few points present epileptic crisis in different brain regions and the associated symptoms.
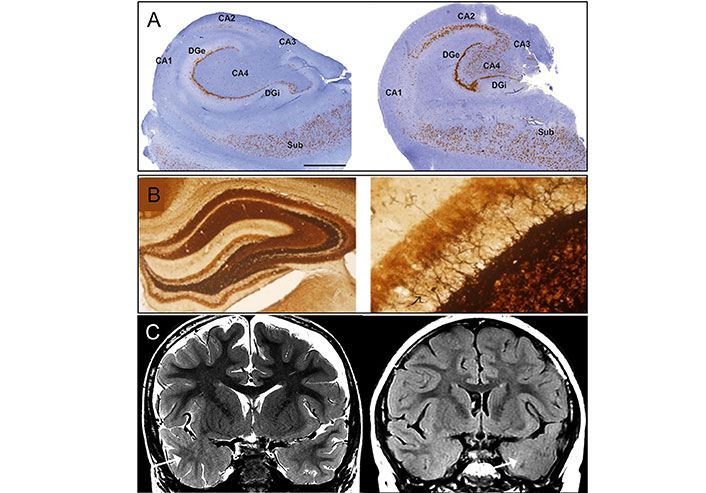
Classical pathology of temporal lobe epilepsy for type I (left) and type II (right). As depicted in the figure labeled A to C, the structure of the hippocampus can be seen at various levels, A being a superresolution level, B mossy fiber sprouting under Timm’s stain in KA-induced epileptic seizure rats, 40× (left) and 400× (right) with back arrow pointing to mossy, and C MRI imaging of focal cortical dysplasia (FCD) in two patients with temporal lobe epilepsy (TLE). Coronal T2-weighted imaging (left), and coronal T2-fluid-attenuated inversion recovery (FLAIR) sequence (right) images through the temporal lobes demonstrating asymmetric hyperintense temporal white matter signal and regional obscuration of corticomedullary interfaces in the temporal tip representing cortical dysplasia (Arrow). DGi: dentate gyrus; Sub: subiculum
Note. Reprinted from “Pathological Targets for Treating Temporal Lobe Epilepsy: Discoveries From Microscale to Macroscale” by You J, Huang H, Chan CTY, Li L. Front Neurol. 2022;12:779558 (https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2021.779558/full). CC BY 4.0. Adapted with permission from “International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: A Task Force report from the ILAE Commission on Diagnostic Methods” by Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, et al. Epilepsia. 2013;54:1315–29 (https://onlinelibrary.wiley.com/doi/10.1111/epi.12220). © 1999-2024 John Wiley & Sons, Inc or related companies; “Electroacupuncture at ST36-ST37 and at ear ameliorates hippocampal mossy fiber sprouting in kainic acid-induced epileptic seizure rats” by Liu CH, Lin YW, Hsu HC, Liu HJ, Lin WJ, Hsieh CL. Biomed Res Int. 2014;2014:756019 (https://onlinelibrary.wiley.com/doi/10.1155/2014/756019). CC BY; “Temporal lobe epilepsy and focal cortical dysplasia in children: A tip to find the abnormality” by Bartolini L, Whitehead MT, Ho CY, Sepeta LN, Oluigbo CO, Havens K, et al. Epilepsia. 2017;58:113–22 (https://onlinelibrary.wiley.com/doi/10.1111/epi.13615). © 1999-2024 John Wiley & Sons, Inc or related companies.
The temporal lobe, notably the mesial temporal structures, like the hippocampus, is frequently implicated in temporal lobe epilepsy. Seizures originating in this lobe can lead to alterations in consciousness and memory disturbances [6]. The hippocampus, which is essential for memory formation and retrieval is implicated in temporal lobe epilepsy with seizures leading to memory impairments and altered consciousness [6].
Frontal lobe epilepsy arises in the frontal lobes of the brain and can manifest as motor movements or unusual behaviors. These seizures often feature brief periods of confusion and automatism [16].
Seizures originating in the parietal lobes can result in sensory disturbances, including tingling, numbness, or unusual sensations in the body [17]. Such that occipital lobe epilepsy involves seizures originating in the occipital lobes, responsible for visual processing. Such seizures can cause visual hallucinations, blindness, or other visual disturbances [18].
To comprehensively explore the pathophysiology of epilepsy in these brain regions, researchers have employed a multi-scale approach that encompasses both mesoscale and macroscale network analyses. These complementary approaches offer unique insights into how localized and global brain networks contribute to epileptic phenomena.
Epilepsy being a network disease, it is beneficial to understand perturbation in the neural network at various levels. The mesoscale network research in epilepsy delves into the intermediate level of neural organization, bridging the gap between microscale neuronal activity and macroscale brain regions. This approach allows for a more nuanced examination of the epileptic brain’s functional and structural connectivity. One notable study [19] employed high-resolution functional magnetic resonance imaging (fMRI) to investigate mesoscale network alterations in patients with epilepsy. Figure 2 shows a mesoscale network connectome from a rat brain with 486 discreet brain regions. The findings revealed aberrant mesoscale connectivity patterns within the default mode network (DMN), providing compelling evidence for network disruption as a key aspect of epilepsy’s pathophysiology. Specifically, they identified increased connectivity within the DMN, which correlated with disease duration and seizure frequency. Such findings underscore the importance of mesoscale network research in unraveling the subtleties of epileptic brain dynamics.
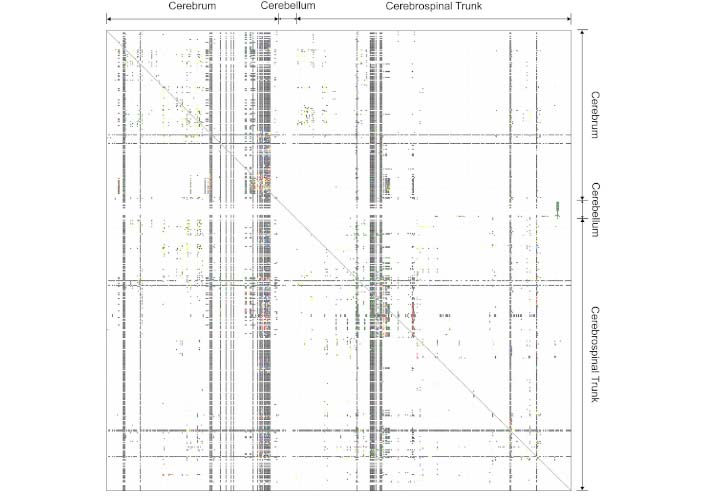
An example of mesoscale network connectome projections between 486 discrete brain regions in the rat brain
Note. Reprinted from “A proposal for a coordinated effort for the determination of brainwide neuroanatomical connectivity in model organisms at a mesoscopic scale” by Bohland JW, Wu C, Barbas H, Bokil H, Bota M, Breiter HC, et al. PLoS Comput Biol. 2009;5:e1000334 (https://journals.plos.org/ploscompbiol/article?id=10.1371/journal.pcbi.1000334). CC BY 4.0.
On the other hand, macroscale network research takes a broader view, focusing on large-scale brain networks and their interactions. The macroscale approach is instrumental in understanding how seizures propagate and affect various brain regions. A seminal study [20] utilized intracranial electroencephalography (EEG) to investigate macroscale network dynamics during seizure events. This research identified critical hubs within the epileptic brain’s macroscale network that play pivotal roles in seizure generation and propagation. Notably, the study revealed that specific brain regions, such as the hippocampus and thalamus, acted as focal points for seizure initiation and dissemination. These findings have profound implications for the development of targeted therapeutic interventions in epilepsy, aimed at modulating macroscale network activity and disrupting seizure spread.
Epileptic seizures, characterized by their paroxysmal and often unpredictable nature, are the defining clinical manifestations of epilepsy. However, beneath the surface of these seizures lies a complex tapestry of electrical events that are equally crucial for understanding, diagnosing, and managing epilepsy. Interictal and ictal spikes are among the most significant electrographic features within this tapestry. They serve as the hidden signatures of epileptic activity, revealing themselves as transient bursts of abnormal electrical discharges amidst the background brain activity. They are crucial for localization of epileptic foci, and warning for predicted seizures [2].
Ictal spikes are distinct electrical events closely related with the active phase of seizure. Their temporal characteristics can vary significantly based on the type and severity of the seizure. While some ictal spikes may be relatively brief, lasting only a few seconds, others can persist for several minutes during more prolonged and intense seizures [21]. Ictal spikes involve extensive portions of the brain. These spikes can propagate across multiple brain regions and exhibit a broader spatial distribution. In some cases, particularly during complex partial seizures or generalized seizures, ictal spikes can affect entire brain hemispheres. The spatial spread of ictal spikes highlights the widespread nature of epileptic activity during seizure events.
Interictal spikes, often referred to as interictal epileptiform discharges are transient electrical events that occur between epileptic seizures. These spikes are relatively short-lived and typically last for a brief period, ranging from a few tens of milliseconds to a few hundred milliseconds [22]. The spatial localization of interictal spikes is a critical aspect of their analysis. These spikes are often highly localized to specific brain regions. Depending on the underlying epileptic focus and the individual’s unique neuroanatomy, interictal spikes exhibit varying spatial extents from a few square millimeters to several centimeters within brain tissue [22]. The spatial distribution of interictal provides valuable information for pinpointing the origin of abnormal electrical activity within the brain.
Understanding the pathophysiology and precise localization of epileptic foci within the brain is paramount for effectively managing the condition. This is where EEG and stereoencephalography (sEEG) are indispensable tools for clinicians and researchers. EEG is a non-invasive technique that serves as a tool for recording and analyzing the electrical activity of the brain. It offers insight into various aspects of brain function, such as cognitive processes, sleep, and abnormal brain activity such as seizures in epilepsy. On the other hand, sEEG is a neurosurgical procedure that involves the placement of depth electrodes directly into or near specific brain regions. It is used for precise localization of neural activity such as the precise region of the epileptic seizure.
EEG typically captures a broad frequency range, including delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), and gamma (30–100 Hz) bands. While sEEG captures similar frequency bands, due to the proximity of sEEG electrodes to neural structures, it can capture more localized and detailed information about neural activity [23]. Higher spatial resolution allows capturing higher-amplitude and more localized seizures. Figure 3 shows a sample of interictal and ictal episodes in a recorded signal where a comparison is shown with a normal recording. It is evident that in the case of an epileptic subject, the spikes are a prominent marker to predict the prognosis of the epileptic event.
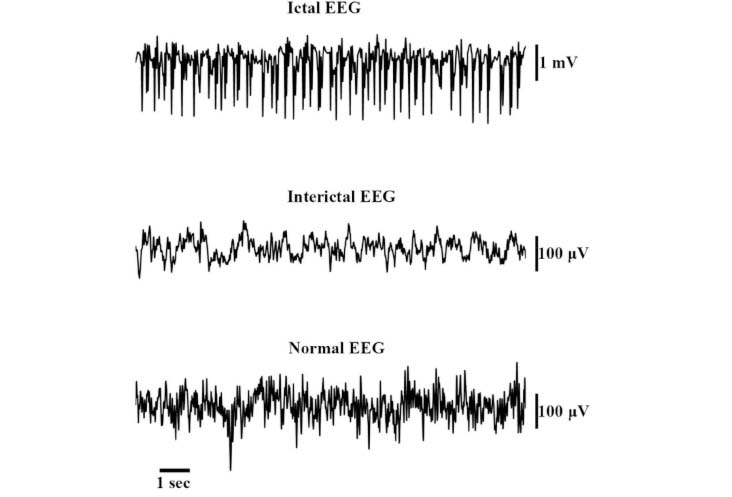
Examples of ictal and interictal spikes in electroencephalography (EEG) compared with normal EEG spikes
Note. Adapted from “Evaluating three different adaptive decomposition methods for EEG signal seizure detection and classification” by Carvalho VR, Moraes MFD, Braga AP, Mendes EMAM. BioRxiv. 2019;691055 (https://www.biorxiv.org/content/10.1101/691055v1). CC BY.
Traditionally, epilepsy research has focused on the physiological basis of seizures and the role of specific brain regions. However, a growing body of evidence suggests that epilepsy should be viewed as a whole brain network-level phenomenon. This perspective recognizes that seizures are not confined to isolated brain areas but involve the coordinated activity of widespread neural networks.
Whole brain network-level analysis considers the brain as a complex, interconnected system, allowing for a more comprehensive understanding of epilepsy. It takes into account the dynamic interactions between different brain regions, the propagation of seizures, and the associated cognitive and behavioral consequences. One way to study whole-brain networks in epilepsy is through computer modeling. Noebels [4] developed a connectome-based brain network model that integrates individual structural and functional data with neural population dynamics. The model was able to predict subjects’ individual resting-state fMRI time series and spatial network topologies over 20 minutes of activity. More importantly, the model also revealed precise neurophysiological mechanisms that underlie and link six empirical observations from different scales and modalities, including slow resting-state fMRI oscillations, spatial topologies of functional connectivity networks, excitation-inhibition balance, pulsed inhibition on short and long time scales, inverse relationships between rhythms, spike-firing, and fMRI on short and long time scales, and fMRI power-law scaling.
The model is considered a whole-brain network-level analysis because it takes into account the interactions between all of the brain regions in the model. The model is able to simulate the propagation of seizures through the brain, as well as the associated changes in brain activity. This allows researchers to study the complex dynamics of epilepsy at the whole-brain network level and it clearly shows the potential of whole-brain network modeling for understanding the complex dynamics of epilepsy. In this pursuit, researchers have utilized a variety of modeling approaches, each with its unique strengths.
Biophysical models aim to simulate the complex interactions among neurons and synapses in the brain. For example, the FitzHugh-Nagumo model, which was originally developed in 1961 by FitzHugh [24], simplifies the behavior of excitable cells using a system of differential equations. It is employed to investigate the mechanisms behind seizure initiation and spread, revealing how changes in the membrane potential of neurons can lead to the generation of ictal spikes and the synchronization of neuronal activity.
Another common biophysical model used to study epilepsy is the Hodgkin-Huxley model [25, 26], which characterizes the generation of action potentials in excitable cells, such as neurons. This model is based on a set of differential equations that describe the voltage-dependent conductance of ion channels, including sodium and potassium channels, within neuronal membranes.
The model simply simulates the behavior of ion channels in neuron membranes, specifically the movement of sodium and potassium ions. It accounts for the changes in membrane potential that underlie action potentials in neurons. Since this model can represent abnormal ion channel behavior or network properties associated with seizures, it can find out altered ionic currents which may lead to changes in neuronal membrane potential, potentially triggering and sustaining epileptic seizures.
Network models, based on graph theory, provide a framework for understanding the connectivity and interactions between brain regions. A study by Wilke et al. [27] employed a network model to represent the connectivity between different brain regions, allowing for the simulation of interactions. This research aimed to investigate the dynamics of epileptic networks, focusing on focal epilepsy. Critical hubs and pathways were identified, shedding light on how surgical resection might be an effective prevention and treatment strategy.
Computational models serves as essential tools in bridging the gap between biophysical and phenomenological models, offering a deeper understanding of the complex dynamics underlying epilepsy in whole brain networks. These models integrate the intricate aspects of neural physiology with the intricate web of network connectivity to simulate the behavior of large-scale brain networks. One exemplary computational model that has significantly contributed to our understanding of epilepsy is the Epileptor model, as introduced by Moosavi et al. [28]. The Epileptor model, inspired by experimental data and neurophysiological insights, allows for the replication of observed EEG patterns during seizures. It underscores the critical role of transitions between different network states in the emergence of epileptic activity, shedding light on the mechanisms that underlie seizure initiation and propagation. The Izhikevich model, proposed by Izhikevich [29] in 2003, is another computational model of neural activity that has found applications in the study of epilepsy. This model provides a balance ance between computational efficiency and biophysical realism. It allows for the simulation of various neuronal firing patterns, such as regular spiking, fast spiking, and bursting. Researchers have leveraged the Izhikevich model to investigate the role of different neuronal types and their interactions in the generation of epileptic activity within whole brain networks. By capturing a wide range of neuronal behaviors, this model helps elucidate the complex dynamics involved in epileptic seizures. Furthermore, the use of graph theory-based models, such as the small-world network model [30], has become increasingly prevalent in the study of epilepsy. These models allow researchers to investigate the topological properties of brain networks and how alterations in network connectivity can contribute to the generation and propagation of seizures.
Figure 4 shows an example of different types of neuronal activity going on in different brain regions.
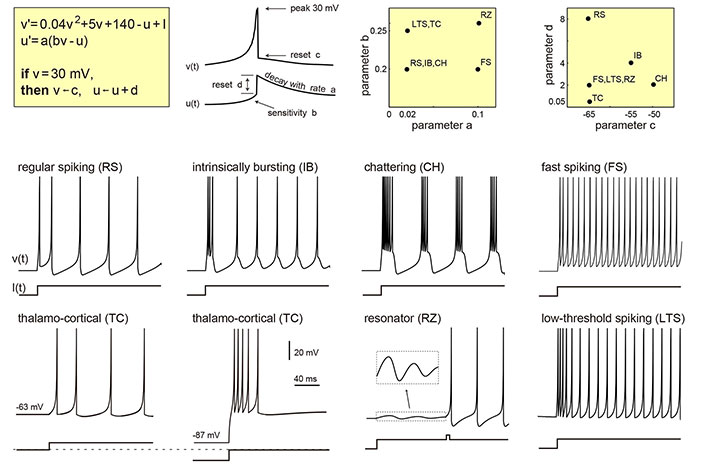
Izhikevich neuron model simulates different types of neurons spiking in various brain regions. The change of the parameter values in the model enables it to mimic the phenomenon
Note. This figure is reproduced with permission from http://www.izhikevich.com. (Electronic version of the figure and reproduction permissions are freely available at http://www.izhikevich.com)
Phenomenological models, like the neural mass model (NMM), offer a higher-level description of system behavior. The NMM, as used by Wendling et al. [31], simulates the collective behavior of neural populations, emphasizing the balance between excitation and inhibition. Figure 5 shows an example of the schematic diagram of the NMM. This model has been employed to understand the dynamics of epileptic networks and how seizures can emerge from altered network states. Another phenomenological model, which is named the Wilson-Cowan model [30] describes the population dynamics of excitatory and inhibitory neurons within a neural network. It simplifies the complex interactions between large groups of neurons and focuses on the balance between excitation and inhibition, which plays a critical role in shaping network-level activity. It is valuable for epilepsy research since it can capture emergent phenomena, such as the synchronization of neural populations during seizures. It provides insights into how changes in the balance of excitation and inhibition can lead to seizure-like activity. Figure 6 shows the structure of the Wilson-Cowan model.
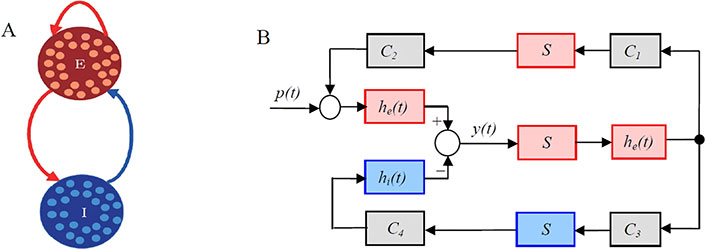
An example of the schematic diagram of the neural mass model (NMM). A. “E” and “I” represent excitatory and inhibitory subpopulations, respectively, that are defined as groups of statistically similar excitatory or inhibitory neurons that share the same inputs and connectivity; B. block diagram of the NMM. The red and blue blocks correspond to excitatory and inhibitory subpopulations, respectively. p(t): is the input of the NMM and modelled by Gaussian noise; C1, C2, C3, and C4 are connectivity constants representing interactions between the subpopulations and characterise the average numbers of synaptic contacts; y(t): the output of the NMM, corresponding to the average synaptic activity of the pyramidal cells, that is measured as the EEG signals. Each subpopulation of the NMM was composed of an excitatory, he(t), or inhibitory synaptic dynamic function, hi(t), and a sigmoid static function, S(v). The synaptic functions, he(t) and hi(t), transform the average pre-synaptic firing rates into average post-synaptic membrane potentials
Note. Reprinted from “Suppressing epileptic activity in a neural mass model using a closed-loop proportional-integral controller” by Wang J, Niebur E, Hu J, Li X. Sci Rep. 2016;6:27344 (https://www.nature.com/articles/srep27344). CC BY 4.0.
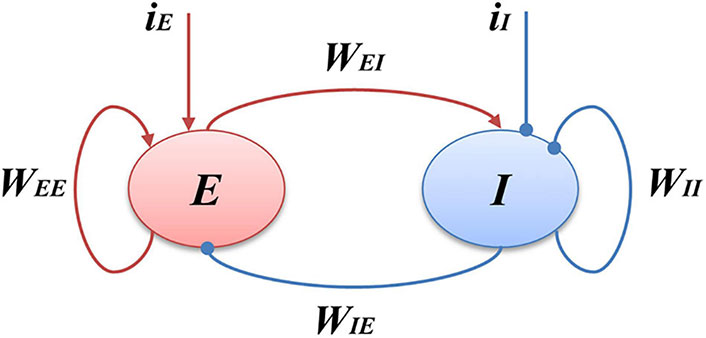
Schematic of the Wilson-Cowan model. E represents the excitatory populations, I stands for the inhibitory populations, the red arrows indicate the excitatory projections and the blue arrows show the inhibitory projections, iI is the external input of the I populations, and iE is the external input of the E populations. WEI and WIE represent the coupling strength between excitatory and inhibitory populations, WEE and WII are the excitatory and inhibitory self-feedback connection strength, respectively. The central idea of this model is to emulate the interaction between the excitatory and inhibitory populations
Note. Reprinted from “Bidirectionally Regulating Gamma Oscillations in Wilson-Cowan Model by Self-Feedback Loops: A Computational Study” by Li X, Li Z, Yang W, Wu Z, Wang J. Front Syst Neurosci. 2022;16:723237 (https://www.frontiersin.org/journals/systems-neuroscience/articles/10.3389/fnsys.2022.723237/full). CC BY 4.0.
In conclusion, employing brain modeling techniques is paramount in advancing our understanding and management of epilepsy for several reasons. Insight into seizure mechanisms: brain modeling allows us to simulate the complex dynamics of epileptic seizures, offering insights into the underlying mechanisms at multiple scales, from cellular interactions to network dynamics. By elucidating the factors that trigger and sustain seizures, modeling contributes to the development of targeted interventions and personalized treatment strategies. Prediction and prevention: computational models of epilepsy enable the prediction of seizure onset and propagation patterns based on individual patient data. This predictive capability holds immense potential for implementing preemptive interventions, such as responsive neurostimulation or pharmacological agents, to abort seizures before they manifest clinically. Optimization of therapeutic approaches: brain models serve as invaluable tools for optimizing existing therapeutic approaches and guiding the development of novel interventions. By simulating the effects of antiepileptic drugs, surgical interventions, or neuromodulation techniques on epileptic networks, modeling facilitates the identification of optimal treatment parameters and patient-specific treatment regimens. Pathophysiological relevance: adding astrocytes can give us more insight into the changes in the network dynamics. Dysfunctional astrocytes contribute to the pathogenesis of various neurological disorders, including epilepsy, Alzheimer’s disease, and mood disorders. Brain modeling with astrocytes enables researchers to simulate disease-specific alterations in astrocytic function and their impact on neuronal circuits, facilitating the identification of novel therapeutic targets and interventions for treating these disorders. Personalized medicine: epilepsy is a heterogeneous disorder with diverse etiologies, seizure types, and treatment responses. Brain modeling allows for the integration of multi-modal patient data, including neuroimaging, electrophysiology, and genetic information, to create personalized computational models of epileptic networks. These models can aid clinicians in tailoring prevention and treatment strategies to individual patients, optimizing efficacy while minimizing side effects. Exploration of novel targets: computational models provide a platform for exploring novel therapeutic targets and interventions for epilepsy. By simulating the effects of manipulating specific cellular or network parameters, modeling can identify potential targets for pharmacological or neuromodulatory interventions, accelerating the discovery and development of novel antiepileptic therapies. Ethical and cost-effective research: brain modeling offers a means to conduct ethically and cost-effectively research in epilepsy. By simulating experimental conditions in silico, researchers can explore hypotheses and test interventions without the need for animal or human subjects, reducing ethical concerns and experimental costs associated with traditional research approaches. In summary, the integration of brain modeling techniques into epilepsy research and clinical practice holds tremendous promise for advancing our understanding of the disorder, optimizing treatment strategies, and ultimately improving outcomes for individuals living with epilepsy. By leveraging computational approaches to simulate epileptic dynamics, predict seizure onset, and guide personalized interventions, brain modeling offers a powerful tool for transforming the management of epilepsy from a reactive to a proactive paradigm.
EEG: electroencephalography
fMRI: functional magnetic resonance imaging
GABA: gamma-aminobutyric acid
NMDA: N-methyl-D-aspartate
NMM: neural mass model
sEEG: stereoencephalography
SB: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. VJ: Validation, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
The first author is thankful for the funding provided by CEA LIST under the Frugal Brain-HAVSENSE [CEA-SI-2022-004] project initiative. This research has received funding from EU’s Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreements [101147319] (EBRAINS2.0 Project). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This does not conflict with reporting this article as an outcome, as part of grants and grants.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Srilaxmi Vityala ... Swathi Nenavath
Joham Choque-Velasquez ... Alder Fernando Valenzuela-Rangel
Rene Ivan Gonzalez-Fernandez ... Jose Luis Hernandez-Caceres
Kabir Sheikh ... Jeffrey Raskin
Eva Žerovnik
Christine Walker, Chris L. Peterson
Darrell O. Ricke
Fumiki Yamashita ... Mari Wataya-Kaneda
Jinwei Zhang
