Affiliation:
AI Technology, Lincoln Laboratory, Massachusetts Institute of Technology, Lexington, MA 02421-6426, USA
Email: Darrell.Ricke@ll.mit.edu
ORCID: https://orcid.org/0000-0002-2842-2809
Explor Neurosci. 2024;3:508–519 DOI: https://doi.org/10.37349/en.2024.00062
Received: August 02, 2024 Accepted: September 04, 2024 Published: October 16, 2024
Academic Editor: Jinwei Zhang, University of Exeter Medical School, UK
The article belongs to the special issue Epilepsy
Aim: Seizure and epilepsy adverse events (AEs) can occur following vaccination. For epilepsy AEs, they are generally expected to only occur at background population frequencies without associations with immunizations. The Vaccine AEs Reporting System (VAERS) collects a subset of AEs experienced by vaccinees, including multiple epilepsy related AEs. This study examines the possibility of immunization associated epilepsy AEs in VAERS occurring above background rates.
Methods: Herein, VAERS is retrospectively examined for epilepsy and seizure AEs following immunizations (AEFIs). Reported AEFIs are normalized by total AEFIs for each vaccine. VAERS data is examined by vaccine type, vaccine source, vaccinee gender, and age-stratified for infants.
Results: Association signals for examined epilepsy and seizure AEFIs are identified for multiple vaccines when compared to other vaccines with normalized frequencies above expected population background frequencies. Normalized epilepsy AEFI frequencies for children less than 1 year are higher than children aged 1 year for several vaccines. For pairs of matched vaccines from different manufacturers, statistically different epilepsy AEFI normalized frequencies were observed. These matched pairs for multiple vaccines implicate likely vaccine contaminations (e.g., endotoxins) as likely candidates for causing elevated epilepsy and seizure AEFIs.
Conclusions: Based on the reported results, delaying some immunizations of a small set of vaccines until children are 1 year of age is predicted to reduce epilepsy AEFI occurrences for these vaccines. For several vaccines, statistically significant differences in epilepsy AEFI normalized frequencies were detected for the same (or similar) vaccine from different manufacturers; this suggests that possible manufacturing contaminant(s) (e.g., endotoxins) as the likely causative agent(s) for observed epilepsy AEFIs above background rates. Eliminating or reducing these possible contaminants is predicted to reduce the observed associations closer to background population levels observed for other vaccines with very low epilepsy AEFI normalized frequencies.
Serious adverse events (SAEs), including seizures and epilepsy AEs, occur in some individuals following vaccination. AEs and SAEs are referred to as AEs following immunization (AEFI). Epilepsy is a neurological disorder that causes recurring seizures. In a given population, observed AEFIs post-vaccination will be represented by a combination of background AEs and (when they occur) vaccine-induced AEs.
Vaccines provide protection against multiple infectious organisms with the risks of SAEs considered in the context of benefits for each vaccine. Two different vaccines targeting the same organism(s) may differ in vaccine type, vaccine components, excipients, and possible manufacturing contaminants; the risks of SAEs for these vaccines may be similar or different.
With respect to epilepsy SAEs, some vaccines and vaccine combinations have been reported more than others. In a five-year retrospective observational study, of the first 245 status epilepticus hospitalizations with immunization records, 14% were vaccine-proximate of which 18 of 35 (51.4%) followed dose-1 measles-mumps-rubella (MMR) vaccine given concomitantly with Haemophilus influenzae type b and meningococcal C conjugate combined vaccine (Hib-MenC), 12 of 35 (34.3%) followed diphtheria-tetanus-acellular pertussis, inactivated polio, H. influenzae type b and hepatitis B combination (DTaP-IPV-Hib-HBV) vaccine in combination with 13-valent pneumococcal conjugate vaccine (PCV13) with 11 of these 12 also given in combination with rotavirus vaccine (RSV), 3 of 35 (8.5%) followed dose-2 MMR-varicella (MMRV) vaccine, 1 of 35 (2.8%) followed Hepatitis A vaccine (HAV), and 1 of 13 (2.8%) followed Varicella zoster vaccine [1]; status epilepticus most commonly occurred with 0–1 days following vaccination or 8–11 days following MMR containing vaccines [1]. Reported SAEs may reflect possible associations above background population frequencies for some vaccines.
One rare type of epilepsy is known to be associated with epilepsy AEFIs triggered by immunization; children with severe myoclonic epilepsy of infancy (Dravet syndrome) can experience epilepsy AEs following vaccination [2]. Mutations in the sodium voltage-gated channel alpha subunit 1 (SCN1A) gene have been observed in 70% of these children [3]. The SCN1A gene encodes a type I voltage-gated sodium channel (Nav 1.1) involved in neural activity [3, 4]. Mutations in the Nav 1.1 channels also cause generalized epilepsy with febrile seizures plus (GEFS+) in older children [4, 5]. In one study, Dravet syndrome patients represented only 2.5% of children with seizures following vaccinations in the first year of life [6]. Protocadherin 19 (PCDH19) [7], gamma-aminobutyric acid type A receptor subunit gamma 2 (GABRG2) [8], and homozygous SCN1B mutations [9] have also been identified (reviewed [10]) in Dravet patients. Note that PCDH19 mutations are associated with either epilepsy with mental retardation limited to females (EFMR) or rarely Dravet syndrome [7]. The currently accepted medical consensus is that aside from children with Dravet syndrome, there are no associations between vaccination and epilepsy AEFIs [11].
Pyrexia (fever) is a common AEFI associated with immunization [12]. Immunizations can cause high fever and rarely febrile seizure AEFIs [13]. Generally considered benign, febrile seizure is the most common convulsive event during childhood [14]. In a study of 109 febrile seizure patients, 6 were diagnosed with subsequent epilepsy 5 to 46 months later of which 3 of 15 had delayed vaccination status (P = 0.03) [15]. In a study of children experiencing seizures, 1.4% of these seizures followed within two weeks of an immunization procedure [16]; all but one of these seizures were associated with fever, often high [16]. One combination vaccine, DTaP-IPV-Hib, was associated with an increased risk of febrile seizures on the day of the first 2 vaccinations [17]. Febrile seizures represent a rare SAE.
When SAE associations exist, it is possible for a vaccine component, excipient, or manufacturing contaminant to be the triggering or causative agent(s). Activation of innate immune responses by Toll-like receptors (TLRs) may play a key role in the etiopathology of epilepsy and convulsive disorders [18]. TLRs are an important family of receptors in the defense against microbes [19]. The TLR4 receptor is activated by lipopolysaccharide (LPS, endotoxin) of Gram-negative bacteria [20]. The bacterial endotoxin lipopolysaccharide enhances seizure susceptibility in mice [21]. One study suggests that vaccination triggers, rather than causes seizures AEFIs [22].
AEFIs are tracked by different countries. In the United States, the Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA), co-sponsor the Vaccine AEs Reporting System (VAERS) database [23]. Medical professionals and vaccinees can report AEFIs to VAERS. The AEFIs reported to VAERS represent a subset of the actual AEFIs experienced by vaccinees (i.e., population samples). Herein, VAERS AEFIs for epilepsy and seizure AEFIs are retrospectively analyzed. This retrospective study examines these AEFIs in the context of two competing hypotheses:
Hypothesis 1—vaccinations are not associated with or trigger epilepsy AEFIs except for individuals with Dravet syndrome.
Hypothesis 2—one or more vaccine manufacturing contaminants, or possibly in some instances vaccine excipients or components, are associated with triggering epilepsy AEFIs for some vaccinees.
Herein, multiple SAE associations are observed for (1) multiple vaccines for infants and (2) human papillomavirus (HPV) vaccines for teenagers and young adults. Statistically different normalized epilepsy AEFIs normalized frequencies from different manufacturers are suggestive of differences resulting from possible manufacturing contaminants (e.g., endotoxins). Predicted manufacturing contaminants (e.g., endotoxins) are consistent with the observed associations with multiple vaccines SAE associations. These observed associations warrant further investigations.
Focusing on epilepsy, the VAERS database [23] was retrospectively examined for the AEs designated by the following Medical Dictionary for Regulatory Activities (MedDRA) codes [24] [MedDRA® the Medical Dictionary for Regulatory Activities terminology is the international medical terminology developed under the auspices of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH)]: epilepsy, infant spasms, petit mal epilepsy, seizure (newer MedDRA code) or convulsion (recently discontinued in VAERS), severe myoclonic epilepsy of infancy (Dravet syndrome), status epilepticus, and generalised tonic-clonic seizure. The downloaded VAERS data includes all AEFIs reported from 1990 to April 26, 2024.
The Python program vaers_reports.py [25] was developed for retrospective analysis of the VAERS data files VAERSDATA, VAERSSYMPTOMS, and VAERSVAX for the years 1990 to 2024 and nondomestic. The vaers_reports.py program tallies vaccine AEFIs by vaccine, day of onset, age, gender, and co-administered vaccines. The vaers_reports.py program takes a list of one or more AE names as input. Note that VAERS vaccine codes are presented in the results and also figures.
In statistics, Pearson correlation is a statistical method that measures the similarity or correlation between two data sets. Herein, Pearson correlation is applied to compare the patterns of AEFI normalized frequencies for multiple vaccines between two AEs. Microsoft Excel was used to calculate Pearson correlations and prepare figures. A Chi-square test is a hypothesis testing method for checking if observed frequencies in one or more categories match expected frequencies. Herein, the Chi-square test is used to examine if the AEFI normalized frequencies (observed AEFI/vaccine AEFIs * constant) are consistent for one AE between two vaccines. Chi-square calculations were performed with an online Chi-square 2 × 2 calculator [26].
Epilepsy AEFIs are expected to only occur at the background rate for matched populations across all vaccines (i.e., very low rates across all vaccines). AE reports to VAERS represent a small subset of AEFIs experienced by all vaccinees. Herein, the frequency of AEFIs is normalized by dividing the observed AEFIs by the total number of all AEFIs for each vaccine and multiplying by 100,000 for an observed normalized frequency per 100,000 vaccinees with any AE. For example, background frequencies for pneumococcal vaccine (PNC) have 4 epilepsy AEFIs out of 28,785 total VAERS reports, which calculates to a normalized frequency of 13 epilepsy AEFIs per 100,000 VAERS reports for PNC. Likewise, influenza vaccine, trivalent (FLU3) has a normalized frequency of 21 per 100,000 VAERS reports for 21 epilepsy AEFIs and 97,636 total VAERS AEFIs for FLU3. These normalized frequencies for PNC and FLU3 likely represent background epilepsy frequencies with expected random variations. Note, that the calculated AEFI normalized frequencies are not adjusted for vaccinees with no symptoms (asymptomatic individuals). With only the following exception, epilepsy AEFIs are expected to represent background population occurrences.
Young vaccinees experiencing epilepsy AEFI may have severe myoclonic epilepsy of infancy (Dravet syndrome); immunization is known to trigger Dravet syndrome in some children [2]. Across all severe myoclonic epilepsy of infancy AEFIs reported in VAERS, only 6VAX-F (diphtheria and tetanus toxoids and acellular pertussis absorbed, inactivated poliovirus, hepatitis B, haemophilus B conjugate vaccine) has a normalized frequency greater than 100 per 100,000 VAERS reports with a minimum of 10 AEFI reported; 6VAX-F has a normalized frequency of 231 per 100,000 VAERS reports which is 8.3 times higher than 28 per 100,000 associated with PNC13 (results provided in [27]). In contrast, thirty vaccines have elevated epilepsy normalized frequencies greater than 100 per 100,000 VAERS reports with a minimum of 10 epilepsy AEFI reports and a minimum of 1,000 vaccine shots with reported AEFIs for each vaccine, see Figure 1. Compared to FLU3 (selected background exemplar), each of these 30 vaccines has a Chi-square P < 0.00001—representing statistically significant elevated AEFI normalized frequencies; these are indicators of vaccine associations above background population rate. For 6VAX-F, the epilepsy AEFI normalized frequency is 9.2 times higher than for severe myoclonic epilepsy of infancy AEFIs. Examining AEFI normalized frequencies by each vaccine manufacturer may detect consistency patterns and/or discordant patterns.
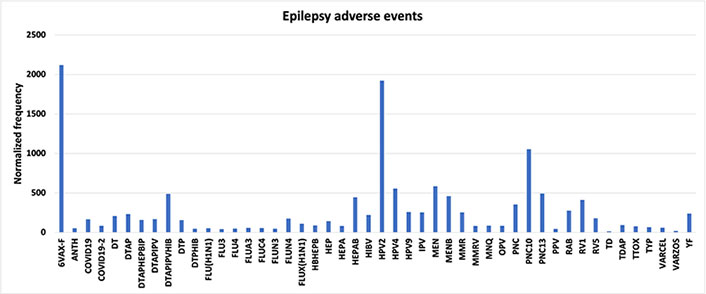
Epilepsy adverse events. Vaccine Adverse Events Reporting System (VAERS) epilepsy adverse events following immunizations (AEFIs) normalized to 100,000 vaccinations with symptoms with a minimum of 1,000 total AEFIs per vaccine. 6VAX-F: diphtheria and tetanus toxoids and acellular pertussis absorbed, inactivated poliovirus, hepatitis B, Haemophilus B conjugate vaccine; ANTH: anthrax vaccine; COVID19: coronavirus 2019 vaccine; DT: diphtheria and tetanus toxoids, pediatric; DTAP: diphtheria-tetanus-acellular pertussis; DTAPHEPBIP: diphtheria and tetanus toxoids and acellular pertussis-hepatitis B-inactivated poliovirus vaccine; DTAPIPV: diphtheria and tetanus toxoids and acellular pertussis-inactivated poliovirus vaccine; DTAPIPVHIB: diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, Haemophilus B conjugate and hepatitis B vaccine; DTP: diphtheria and tetanus toxoids and pertussis vaccine; DTPHIB: diphtheria and tetanus toxoids and pertussis-Haemophilus B conjugate vaccine; FLU: influenza vaccine; HBHEPB: Haemophilus B conjugate-hepatitis B vaccine; HEP: hepatitis B vaccine; HIBV: Haemophilus B conjugate vaccine; HPV2: human papillomavirus 2; IPV: inactivated polio vaccine; MEN: meningococcal polysaccharide vaccine; MENB: meningococcal group B vaccine; MMRV: measles-mumps-rubella varicella; MNQ: meningococcal conjugate vaccine; OPV: oral poliovirus vaccine trivalent, live; PNC: pneumococcal vaccine; PPV: pneumococcal vaccine, polyvalent; RAB: rabies virus vaccine; RV1: rotavirus vaccine, live, oral; TD: tetanus and diphtheria toxoids, adult; TDAP: tetanus, diphtheria, and pertussis; TTOX: tetanus toxoid; TYP: typhoid vaccine; VARCEL: varivax-varicella virus live; VARZOS: varicella-zoster vaccine; YF: yellow fever vaccine
Some vaccines have more than one manufacturer. For these vaccines, observed epilepsy AEFIs normalized frequencies for individual vaccines can be similar or highly discordant (Figure 2): DTAP: Infanrix vs. Tripedia has Chi-square P < 0.00001; tetanus, diphtheria, and pertussis (TDAP): Adacel vs. Boostrix P = 0.000015; DTaP-IPV-Hib: Infanrix Quinta vs. Pentacel P < 0.00001; Hepatitis B (HEPB): Recombivax HB vs. Foreign P < 0.00001 and vs. Engerix-B P = 0.000778; HIB: Hibtiter vs. Acthib P < 0.00001, vs. Hiberix P < 0.00001, and vs. Pedvaxhib P = 0.026239; MMR: Virivac vs. Priorix P = 0.000016; Meningococcal B: Bexsero vs. Trumenba P = 0.000125; Pneumo: Pneumovax vs. Prevnar, Prevnar13, and Synflorix all with P < 0.00001; IPV: Ipol vs. Poliovax P = 0.022385; Rotavirus: Rotarix vs. Rotateq P = 0.000054. This data is inconsistent with hypothesis 1, and hypothesis 1 needs to be rejected or revised. This data is supportive of hypothesis 2 for one or more manufacturing contaminants triggering epilepsy AEFIs in some vaccinees. Are some vaccinees possibly more susceptible to these proposed contaminants than other vaccinees?
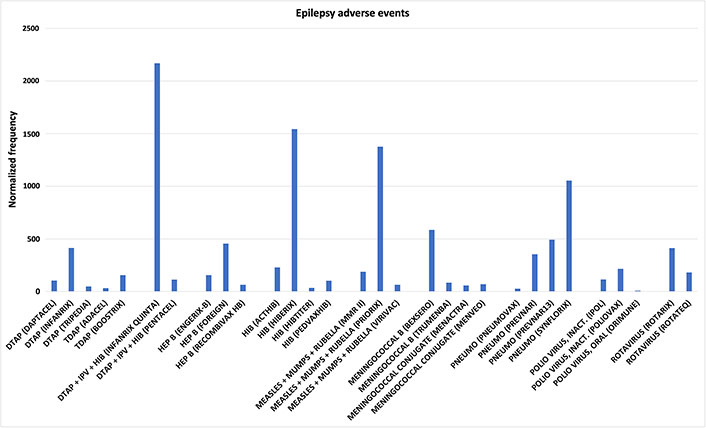
Epilepsy adverse events. Epilepsy adverse events following immunizations (AEFIs) normalized to 100,000 vaccinations with symptoms for vaccines with at least two sources; normalized frequencies for vaccines with less than 1,000 Vaccine Adverse Events Reporting System (VAERS) reports are not shown (full data available [27]). DTAP: diphtheria-tetanus-acellular pertussis; TDAP: tetanus, diphtheria, and pertussis; IPV: inactivated polio vaccine; HIB: Haemophilus influenzae type b; HEP B: hepatitis B
Comparing age-stratified normalizing AEFIs can provide possible insights. Normalized frequencies of epilepsy AEFIs for infants are illustrated in Figure 3 for multiple vaccines for infants aged 0 and 1; these AEFIs could include events triggering Dravet syndrome for a small subset of these individuals. Note that the frequencies of epilepsy AEFIs for infants age 0 are generally higher than for infants age 1 (Figure 3). Note that the CDC does not recommend MMR immunization until infants are one year old [28].
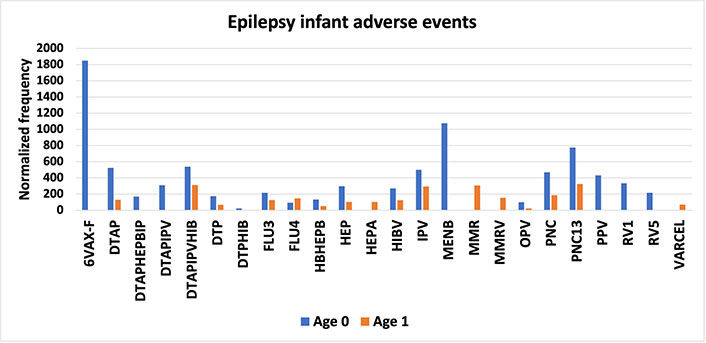
Epilepsy infant adverse events. Vaccine Adverse Events Reporting System (VAERS) epilepsy adverse events following immunizations (AEFIs) normalized to 100,000 vaccinations with symptoms for ages 0 and 1; normalized frequencies for vaccines by age with less than 1,000 VAERS reports are not shown (full data available [27]). 6VAX-F: diphtheria and tetanus toxoids and acellular pertussis absorbed, inactivated poliovirus, hepatitis B, Haemophilus B conjugate vaccine; DTAP: diphtheria-tetanus-acellular pertussis; DTAPHEPBIP: diphtheria and tetanus toxoids and acellular pertussis-hepatitis B-inactivated poliovirus vaccine; DTAPIPV: diphtheria and tetanus toxoids and acellular pertussis-inactivated poliovirus vaccine; DTAPIPVHIB: diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, Haemophilus B conjugate and hepatitis B vaccine; DTP: diphtheria and tetanus toxoids and pertussis vaccine; DTPHIB: diphtheria and tetanus toxoids and pertussis-Haemophilus B conjugate vaccine; FLU3: influenza vaccine, trivalent; HBHEPB: Haemophilus B conjugate-hepatitis B vaccine; HEP: hepatitis B vaccine; HIBV: Haemophilus B conjugate vaccine; IPV: inactivated polio vaccine; MENB: meningococcal group B vaccine; MMR: measles-mumps-rubella; MMRV: MMR varicella; OPV: oral poliovirus vaccine trivalent, live; PNC: pneumococcal vaccine; PPV: pneumococcal vaccine, polyvalent; RV1: rotavirus vaccine, live, oral; VARCEL: varivax-varicella virus live vaccine
For older children, teenagers, and adults, the HPV vaccine is provided to all genders for protection against human papilloma virus associated cervical cancer in women. For HPV vaccinees, ages 9 years old and above, the AEFI normalized frequencies for these individuals are inconsistent with Dravet syndrome associations. It is possible that one or more manufacturing contaminants, vaccine components, or possibly vaccine excipients are associated with triggering epilepsy AEFIs in these older individuals, consistent with hypothesis 2.
VAERS includes additional MedDRA categories for specific types of epilepsy that were examined. Normalized frequencies for VAERS AEFIs generalized tonic-clonic seizure, petit mal epilepsy, and status epilepticus are illustrated in Figure 4. Infant spasms or West syndrome is considered a spectrum of disorders named infantile spasm syndrome (ISs) [29]. Infantile spasms have a small prevalence in males [29]; inconsistent with this known gender bias, normalized frequencies for infantile spams reported to VAERS are essentially equivalent for both genders (Figure 5). The vaccines with higher infantile spasms AEFIs overlap with epilepsy AEFIs (Figures 3 and 5) and generalized tonic-clonic seizure, petit mal epilepsy, and status epilepticus (Figures 4 and 5).
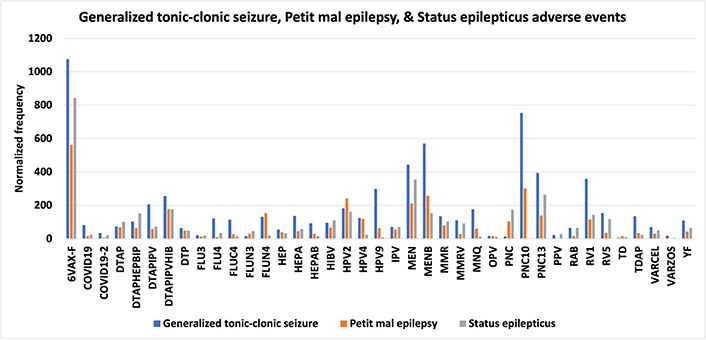
Generalized tonic-clonic seizure, petit mal epilepsy, and status epilepticus adverse events. Vaccine Adverse Events Reporting System (VAERS) generalized tonic-clonic seizure, petit mal epilepsy, and status epilepticus adverse events following immunizations (AEFIs) normalized to 100,000 vaccinations with symptoms (only 2 vaccines included for PNC10); normalized frequencies for vaccines with less than 1,000 VAERS reports are not shown (full data available [27]). 6VAX-F: diphtheria and tetanus toxoids and acellular pertussis absorbed, inactivated poliovirus, hepatitis B, Haemophilus B conjugate vaccine; COVID19: coronavirus 2019 vaccine; DTAP: diphtheria-tetanus-acellular pertussis; DTAPHEPBIP: diphtheria and tetanus toxoids and acellular pertussis-hepatitis B-inactivated poliovirus vaccine; DTAPIPV: diphtheria and tetanus toxoids and acellular pertussis-inactivated poliovirus vaccine; DTAPIPVHIB: diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, Haemophilus B conjugate and hepatitis B vaccine; DTP: diphtheria and tetanus toxoids and pertussis vaccine; FLU3: influenza vaccine, trivalent; HEP: hepatitis B vaccine; HIBV: Haemophilus B conjugate vaccine; HPV2: human papillomavirus 2; IPV: inactivated polio vaccine; MEN: meningococcal polysaccharide vaccine; MMR: measles-mumps-rubella; MMRV: MMR varicella; MNQ: meningococcal conjugate vaccine; OPV: oral poliovirus vaccine trivalent, live; PNC: pneumococcal vaccine; PPV: pneumococcal vaccine, polyvalent; RAB: rabies virus vaccine; RV1: rotavirus vaccine, live, oral; TD: tetanus and diphtheria toxoids, adult; TDAP: tetanus, diphtheria, and pertussis; VARCEL: varivax-varicella virus live; VARZOS: varicella-zoster vaccine; YF: yellow fever vaccine
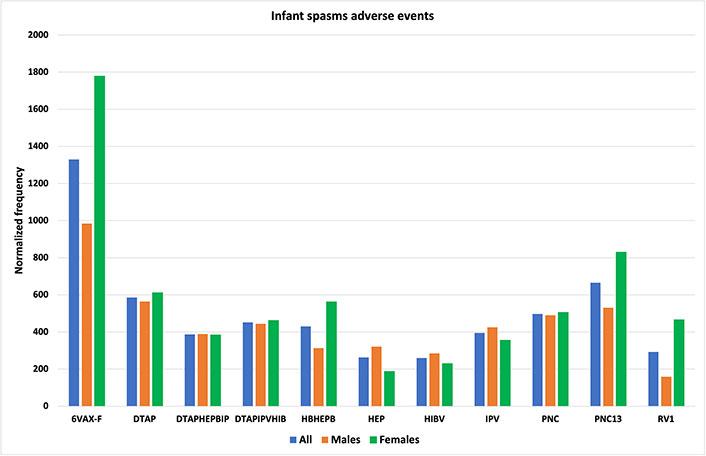
Infant spasms adverse events. Vaccine Adverse Events Reporting System (VAERS) infant spasms adverse events following immunizations (AEFIs) normalized to 100,000 vaccinations with symptoms for infants age 0; normalized frequencies for vaccines with less than 1,000 VAERS reports are not shown (full data available [27]). 6VAX-F: diphtheria and tetanus toxoids and acellular pertussis absorbed, inactivated poliovirus, hepatitis B, Haemophilus B conjugate vaccine; DTAP: diphtheria-tetanus-acellular pertussis; DTAPHEPBIP: diphtheria and tetanus toxoids and acellular pertussis-hepatitis B-inactivated poliovirus vaccine; DTAPIPVHIB: diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, Haemophilus B conjugate and hepatitis B vaccine; HBHEPB: Haemophilus B conjugate-hepatitis B vaccine; HEP: hepatitis B vaccine; HIBV: Haemophilus B conjugate vaccine; IPV: inactivated polio vaccine; PNC: pneumococcal vaccine; RV1: rotavirus vaccine, live, oral
Single seizure AEFIs are reported separately in VAERS from epilepsy AEFIs; these AEFIs are analyzed for comparison to the epilepsy AEFIs. Normalized frequencies for (single) seizure AEFIs are shown in Figure 6. A possible similar pattern of AEFI normalized frequencies for seizure AEFIs (Figure 6) has a Pearson correlation of r = 0.37 compared to epilepsy AEFIs (Figure 1); this correlation is consistent with a possible common etiology between these two AEs (e.g., triggering by possible manufacturing contaminants—endotoxins).
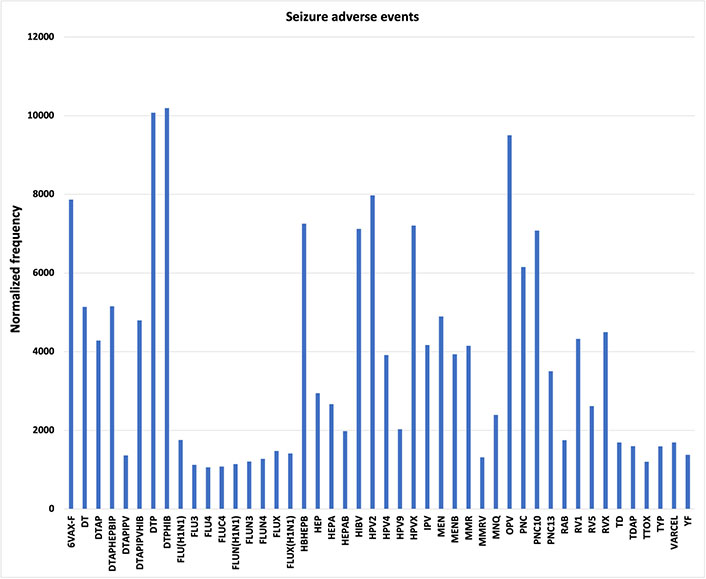
Seizure adverse events. Vaccine Adverse Events Reporting System (VAERS) (single) seizure adverse events following immunizations (AEFIs) normalized to 100,000 vaccinations with symptoms; normalized frequencies for vaccines with less than 1,000 VAERS reports are not shown (full data available [27]). 6VAX-F: diphtheria and tetanus toxoids and acellular pertussis absorbed, inactivated poliovirus, hepatitis B, Haemophilus B conjugate vaccine; DT: diphtheria and tetanus toxoids, pediatric; DTAP: diphtheria-tetanus-acellular pertussis; DTAPHEPBIP: diphtheria and tetanus toxoids and acellular pertussis-hepatitis B-inactivated poliovirus vaccine; DTAPIPV: diphtheria and tetanus toxoids and acellular pertussis-inactivated poliovirus vaccine; DTAPIPVHIB: diphtheria and tetanus toxoids and acellular pertussis, inactivated poliovirus, Haemophilus B conjugate and hepatitis B vaccine; DTP: diphtheria and tetanus toxoids and pertussis vaccine; DTPHIB: diphtheria and tetanus toxoids and pertussis-Haemophilus B conjugate vaccine; FLU: influenza vaccine; HBHEPB: Haemophilus B conjugate-hepatitis B vaccine; HEP: hepatitis B vaccine; HIBV: Haemophilus B conjugate vaccine; HPV2: human papillomavirus 2; IPV: inactivated polio vaccine; MEN: meningococcal polysaccharide vaccine; MENB: meningococcal group B vaccine; MMR: measles-mumps-rubella; MMRV: MMR varicella; MNQ: meningococcal conjugate vaccine; OPV: oral poliovirus vaccine trivalent, live; PNC: pneumococcal vaccine; RAB: rabies virus vaccine; RV1: rotavirus vaccine, live, oral; TD: tetanus and diphtheria toxoids, adult; TDAP: tetanus, diphtheria, and pertussis; TTOX: tetanus toxoid; TYP: typhoid vaccine; VARCEL: varivax-varicella virus live vaccine; YF: yellow fever vaccine
The current accepted medical consensus is consistent with hypothesis 1 with no associations of epilepsy AEs with vaccinations (aside from trigger event associations for children with Dravet syndrome) [11]. For some vaccines, the retrospective AEFIs in VAERS for epilepsy are discordant with hypothesis 1; some vaccines (6VAX-F, HPV2, PNC10, and others) have high epilepsy AEFI normalized frequencies above background levels observed for other vaccines. For the same vaccine from different manufacturers, the normalized frequencies are highly discordant with statistical significance (Figure 2). In addition, epilepsy AEFIs associated with HPV vaccinations in older individuals are unlikely vaccinees with Dravet syndrome. Hence, hypothesis 1 is rejected.
Many of the vaccines associated with higher epilepsy AEFIs normalized frequencies (Figure 1) are also associated with generalized tonic-clonic seizure, petit mal epilepsy, status epilepticus, infant spasms, and seizure AEFIs (Figures 4, 5 and 6). While shared vaccine excipients have not been excluded, possible shared manufacturing contaminants are more likely associated with these observed vaccine AEFI associations. While inclusion of bacterial vaccine components [Bordetella pertussis (DTaP, 6VAX-F, etc.), H. influenzae (Hib), Streptococcus pneumoniae (PNC), Neisseria meningitidis (meningococcal—MenB), etc.] is a possible factor, large differences in normalized frequencies for the same vaccine from different manufacturers (Figure 2) supports possible shared manufacturing contaminants as the more probable risk factor. Note that infants age 0 have higher susceptibility to these risks than older infants age 1 (Figure 3).
Generalized tonic-clonic seizure AEFIs, petit mal epilepsy AEFIs, and status epilepticus AEFIs exhibit similar associations as epilepsy AEFIs for multiple vaccines, with the highest normalized frequencies for 6VAX-F for these three AEFIs (Figure 4). Forty-six seizure AEFI normalized frequencies (Figure 6) are above 1,000 per 100,000 VAERS reports.
The VAERS database collects only a small subset of AEFIs experienced by vaccinees. Any reporting biases or exclusion of AEFIs would perturb the normalized frequencies presented herein. The AEFI normalized frequencies estimated for vaccinees with any AEs and do not include asymptomatic individuals; these AEFI normalized frequencies can be adjusted by adjusting the population size (P) to include the population fraction of asymptomatic vaccinees.
The results from this study suggest multiple possible follow-up studies. First, given that epilepsy AEFIs are relatively rare, it is suggested to acquire comparable results to those presented herein by retrospective analysis of other vaccine AEs databases. Second, examining some of the detected safety signals for identified vaccines with elevated AEFI normalized frequencies could be included as part of ongoing or future clinical studies. Third, infants age 0 have higher normalized epilepsy AEFI than infants age 1 for multiple vaccines (Figure 3). Delaying immunization of some of these vaccines for infants aged 0 until slightly older is predicted to lower the epilepsy AEFI occurrences for infants. Fourth, the normalized frequencies presented support the hypothesis that one or more manufacturing contaminants are acting as triggers for epilepsy, generalized tonic-clonic seizure, petit mal epilepsy, status epilepticus, infant spasms, and seizure AEFIs. The most likely manufacturing contaminants for observed associations are endotoxins. Evaluating vaccine lot samples with mass spectrometry (or other quality control techniques) focusing on paired identical vaccines with highly discordant epilepsy AEFIs normalized frequencies (Figure 2) is recommended for quantifying possible manufacturing contaminants. Fifth, developing an animal model for epilepsy AEFIs may be highly informative to understanding the etiology in humans. Understanding the etiology of these AEFIs may lead to procedures to minimize their occurrences.
The VAERS database was retrospectively examined for epilepsy and related AEFIs. Normalized frequencies identify associations for these AEFIs for multiple vaccines. These AEFIs may be triggered by possible manufacturing contaminants (e.g., endotoxins) administered within these vaccines. Eliminating or reducing these possible contaminants may reduce the observed associations closer to background population levels.
6VAX-F: diphtheria and tetanus toxoids and acellular pertussis absorbed, inactivated poliovirus, hepatitis B, Haemophilus B conjugate vaccine
AEFIs: adverse events following immunizations
AEs: adverse events
DTaP: diphtheria-tetanus-acellular pertussis
FLU3: influenza vaccine, trivalent
Hib: Haemophilus influenzae type b
HPV: human papillomavirus
IPV: inactivated polio vaccine
MedDRA: Medical Dictionary for Regulatory Activities
MMR: measles-mumps-rubella
PNC: pneumococcal vaccine
SAEs: serious adverse events
SCN1A: sodium voltage-gated channel alpha subunit 1
TLRs: Toll-like receptors
VAERS: Vaccine Adverse Events Reporting System
MedDRA® trademark is registered by ICH.
DOR: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. The author has read and approved the submitted version.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The datasets analyzed for this study open data in Harvard Dataverse [27].
Distribution Statement: Approved for public release. Distribution is unlimited. This material is based upon work supported by the Department of the Air Force under Air Force Contract [No. FA8702-15-D-0001]. Any opinions, findings, conclusions, or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the Department of the Air Force. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Srilaxmi Vityala ... Swathi Nenavath
Joham Choque-Velasquez ... Alder Fernando Valenzuela-Rangel
Rene Ivan Gonzalez-Fernandez ... Jose Luis Hernandez-Caceres
Kabir Sheikh ... Jeffrey Raskin
Eva Žerovnik
Christine Walker, Chris L. Peterson
Swati Banerjee, Viktor Jirsa
Fumiki Yamashita ... Mari Wataya-Kaneda
Jinwei Zhang
