Affiliation:
Human Cytogenetics and Genomics Laboratory, Faculty of Allied Health Sciences, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Chennai 603103, Tamil Nadu, India
ORCID: https://orcid.org/0009-0008-5346-9597
Affiliation:
Human Cytogenetics and Genomics Laboratory, Faculty of Allied Health Sciences, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Chennai 603103, Tamil Nadu, India
ORCID: https://orcid.org/0009-0003-2904-5421
Affiliation:
Human Cytogenetics and Genomics Laboratory, Faculty of Allied Health Sciences, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Chennai 603103, Tamil Nadu, India
Email: rkgenes@gmail.com
ORCID: https://orcid.org/0000-0002-9307-5428
Explor Neuroprot Ther. 2024;4:308–318 DOI: https://doi.org/10.37349/ent.2024.00085
Received: March 08, 2024 Accepted: June 17, 2024 Published: July 14, 2024
Academic Editor: Abdelhamid Benazzouz, Bordeaux University, France
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by both non-motor and motor symptoms, due to the loss of dopamine-producing neurons in the brain. Monoamine oxidase-B (MAO-B) inhibitors are essential in the treatment of PD, as they increase dopamine levels and could potentially slow down the progression of the disease. MAO-B inhibitors block the ability of the enzyme to degrade dopamine in the brain. MAO-B inhibitors work by inhibiting this enzyme, which raises dopamine levels and helps reduce motor symptoms, such as akinesia and stiffness in the muscles. In addition to their impact on dopamine levels, MAO-B inhibitors may possess neuroprotective properties. Research indicates that these inhibitors can shield neurons from the harmful byproducts of dopamine breakdown, such as dihydroxy acetaldehyde and hydrogen peroxide. This neuroprotective effect could potentially slow the progression of PD and protect against neuronal damage. MAO-B inhibitors are effective in treating both advanced and early stages of PD. They are recommended as initial treatments for individuals with early PD and can also be used as supplementary therapy in advanced PD to assist in managing motor complications. Additionally, MAO-B inhibitors have shown promise for the treatment of non-motor symptoms of PD, such as fatigue and sleep disturbances. MAO-B inhibitors are an important class of drugs for the treatment of PD, offering both symptomatic relief and potential disease-modifying effects. The goal of ongoing research and development of MAO-B inhibitors is to enhance their safety and selectivity profiles, which could lead to improved treatment approaches for PD and other neurodegenerative disorders.
Parkinson’s disease (PD) is a disorder caused by the loss of neurons that produce dopamine in the midbrain, leading to decreased levels of dopamine in the basal ganglia [1]. The lack of dopamine can lead to a range of movement issues, such as akinesia, bradykinesia, muscle stiffness, and resting tremors, which are collectively referred to as “parkinsonism” [2]. Figure 1 shows the pathway of PD.
Significant neuronal dysfunction outside the basal ganglia circuitry can occur in PD, leading to non-motor symptoms, such as mood and psychiatric disorders. These symptoms often manifest well in advance of actual motor dysfunction [3]. The main goal of PD treatment over the past 50 years has been to control basal ganglia dopamine levels through the administration of levodopa. The central nervous system (CNS) uses levodopa, a precursor of dopamine, to produce dopamine. For the time being, levodopa is the most effective treatment for motor symptoms. However, a few issues are associated with chronic usage. Owing to peripheral rather than central conversion to dopamine in the brain, prolonged levodopa therapy frequently results in significant side effects [4]. After receiving levodopa therapy for five to seven years, dyskinesia is thought to affect 30% to 80% of patients [5].
Dopamine agonists (DAs) are frequently used to treat motor complications. This includes the use of monoamine oxidase-B (MAO-B) and catechol-O-methyltransferase (COMT) inhibitors. MAO-B inhibitors function by blocking central MAO activity in the CNS, thereby reducing the breakdown of dopamine. These inhibitors can cross the blood-brain barrier to be effective [6]. In addition to being very safe and effective in the initial stages of PD, MAO-B inhibitors have shown promise as an adjuvant treatment in later stages of the disease [7, 8]. Research has demonstrated that extended exposure to MAO-B inhibitors is associated with a reduction in the rate of clinical decline and a lower need for levodopa in patients with PD [9]. Currently, approved MAO-B inhibitors for PD include irreversible inhibitors, such as rasagiline and selegiline [10]. This review examines how PD symptoms, both motor and non-motor, are affected by MAO-B inhibitors. As we discuss the development of MAO-B inhibitors for PD treatment, we will delve into their mechanisms of action.
On the outer membrane of mitochondria lies the riboflavin protein MAO, which contributes to the oxidative deamination of neurotransmitters such as dopamine and norepinephrine, as well as monoamine and tyramine [11]. There are two different forms of MAO, MAO-A and MAO-B. The heart, platelets, and gastrointestinal system are primarily found in MAO-A. The main cell types containing MAO-B are glial cells and platelets. Within the brain, total MAO activity consists of approximately 80% MAO-B and 20% MAO-A [12]. MAO-B inhibitors inhibit MAO-B activity in the brain. This leads to the blockade of dopamine breakdown. This action results in the enhancement of dopamine signaling [13]. In the in vivo rat experiments, preclinical studies have shown that rasagiline, a second-generation MAO-B inhibitor, is up to 15 times more effective than selegiline [14].
The ability of a specific medication to reduce, halt, or even reverse the disintegration of dopaminergic cells in the substantia nigra is referred to as “disease modification” in PD patients. The objective of this procedure is to delay the clinical advancement of the disease [15]. The metabolism of dopamine by MAO-B produces toxic byproducts, including dihydroxyacetaldehyde and hydrogen peroxide. MAO-B inhibitors have the potential to protect neurons by blocking these detrimental mechanisms [16]. It is believed that the propargylamine structures of selegiline and rasagiline, which function independently of their inhibition of MAO-B, are responsible for their neuroprotective effects [17]. Rasagiline and selegiline are two drugs that can increase the expression levels of nerve growth factors and brain-derived neurotrophic factors, contributing to their neuroprotective effects [18]. Rasagiline and selegiline are two drugs that may provide neuroprotection. However, they differ in their metabolic pathway. For instance, when tested on PC12 cell lines, both drugs reduced the cell death caused by oxygen-glucose deprivation. However, in contrast to the amino primary metabolite produced by rasagiline, L-methamphetamine enhanced cell death caused by oxygen-glucose deprivation by 70% [19] (Figure 2).
Mitochondrial dysfunction, characterized by increased reactive oxygen species (ROS) generation and impaired mitochondrial complex I activity, is associated with PD and contributes to dopaminergic cell death in the brain [20]. In vitro studies by Czerniczyniec et al. [21] suggest that MAO-B inhibitors can enhance brain mitochondrial function by reducing hydrogen peroxide (a type of ROS) generation and blocking nitric oxide synthase activity, which collectively contributes to oxidative stress and mitochondrial damage. These studies indicate that maintaining mitochondrial membrane potential (ΔΨm) with in vivo deprenyl treatment (a MAO-B inhibitor) can prevent mitochondria from undergoing calcium-induced permeability transition, a critical step in the initiation of cell death pathways. This suggests that modifying MAO activity, particularly using MAO-B inhibitors, could potentially reduce mitochondrial oxidative stress and alter the progression of neurodegenerative diseases like PD. By reducing ROS generation and maintaining mitochondrial function, these inhibitors may help protect dopaminergic cells from death. The MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) model, mentioned in the studies, serves as a well-known experimental model of PD. MPTP is converted to MPP+ (1-methyl-4-phenylpyridinium) by MAO-B, and MPP+ inhibits mitochondrial complex I, ultimately leading to cell death.
Lewy body formation is heavily dependent on alpha-synuclein (AS). In vitro studies have shown that MAO-B inhibitors can postpone the nucleation phase of AS aggregation, potentially impeding the disease’s progression [22]. AS is encouraged by MAO-B inhibitors to form dimeric structures while preventing the formation of linear structures and sheet-like structures necessary for aggregation [23]. MAO-B inhibitors have confirmed efficacy in treating both the initial and advanced stages of PD and may have the potential to modify the disease due to their various pharmacological mechanisms (Table 1).
MAO-B inhibitors used to treat PD-related symptoms
| Symptoms | Authors & year | Results | MAO inhibitors | Reference |
|---|---|---|---|---|
| Motor symptoms | ||||
| Monotherapy in early PD | Mizuno et al., (2017) | The study showed improvements in UPDRS scores. | Selegiline | [26] |
| Supplementary treatment for severe PD | Zhang et al., (2018) | According to the study, there was a reduction in the average amount of time per day that individuals with PD experienced “OFF” periods. | Rasagiline | [31] |
| Non-motor symptoms | ||||
| Sleep discomfort | Gallazzi et al., (2021) | The study demonstrated a noteworthy enhancement in drowsiness. | Selegiline | [33] |
| Fatigue symptoms | Lim et al., (2015) | According to the study, there was a significant improvement in the scores of the Modified Fatigue Impact Scale. | Rasagiline | [35] |
MAO-B: monoamine oxidase-B; PD: Parkinson’s disease; UPDRS: Unified Parkinson’s Disease Rating Scale
In 2017, the National Institute for Health and Care Excellence (NICE) recommended MAO-B inhibitors as the first-line treatment for patients with PD who were not at the end of their life due to motor symptoms. In addition to levodopa, patients with PD who exhibit dyskinesia or motor fluctuations may also be prescribed MAO-B inhibitors [24].
Selegiline and rasagiline, MAO-B inhibitors, are effective monotherapies for early-stage PD. Nevertheless, rasagiline appears to be a therapeutically useful adjuvant to DA medication for early PD, whereas safinamide is not. There is insufficient data to support the use of selegiline for treating motor fluctuations; however, both rasagiline and safinamide work well. Compared to those assigned DAs, patients treated with MAO-B inhibitors had a considerably higher chance of discontinuing their assigned medication class during levodopa-sparing therapy. The primary reason for discontinuation was side effects, with a small percentage attributed to a lack of efficacy [25]. Although both DAs and MAO-B inhibitors are used to treat PD, DAs are generally considered more effective and have a higher risk of side effects. The PD MED study discovered that when used as the first levodopa-sparing medication, MAO-B inhibitors were at least as effective as DAs.
In individuals with early PD, a double-blind study found that selegiline monotherapy may halt disease progression and postpone the need for levodopa. In one study, selegiline delayed the need for levodopa by 548.9 days while those receiving a placebo experienced a delay of only 312.1 days. A 2017 study involving 292 Japanese patients with early PD found that selegiline monotherapy significantly improved their Unified Parkinson’s Disease Rating Scale (UPDRS) scores [26].
In patients with early-stage PD, rasagiline can change the condition and alleviate motor symptoms. A 2002 study assessed the effectiveness of rasagiline in treating patients with early PD in two countries. Rasagiline monotherapy resulted in an adjusted effect size for the overall UPDRS after 26 weeks of treatment of 4.20 units improvement [27].
The study results showed that selegiline and vitamin E combination therapy delayed PD progression and reduced the need for levodopa in early PD patients [28]. Individuals with early-stage PD who did not respond well to their current DA treatment were selected for the study. They were randomly assigned to receive either a placebo or 1 mg of rasagiline. The study demonstrated that adjunct therapy with rasagiline led to an increase in the UPDRS motor score and overall UPDRS score from baseline to week 18 compared to placebo. Importantly, rasagiline did not have any significant side effects [29].
Several studies have examined the effectiveness of selegiline as an additional treatment for patients with advanced PD. One such study involved 96 patients with PD with noticeable variations in symptoms over 6 weeks. The group that was administered 5 mg of selegiline showed a higher percentage of individuals with improved gait and a greater improvement in symptom scores [30]. In 2018, a study investigated the effectiveness of rasagiline as an adjuvant for Chinese patients with motor fluctuations after levodopa administration. The study found that the use of rasagiline significantly decreased the mean total daily “OFF” time compared to a placebo. This treatment was well received by the patients and helped to improve their overall health and daily functioning [31].
Multiple types of non-motor symptoms, including fatigue and sleep disturbances, can arise at any stage of PD.
Non-motor symptoms of subjective sleep discomfort affect approximately 50% of newly diagnosed PD patients [32]. Patients who experience extreme daytime sleepiness may attempt selegiline. Gallazzi et al. [33] found that 45 PD patients who developed excessive daytime sleepiness after being introduced to DAs with 10 mg of selegiline were well-received. Despite a statistically significant improvement in somnolence, the UPDRS scores did not change [33]. When administered as an adjunct therapy, rasagiline helps patients with PD sleep better. For example, the adjunct rasagiline to levodopa increased mean total sleep time, reduced mean sleep latency compared to levodopa alone, and improved sleep outcomes compared to baseline in a 12-week prospective observational trial [34].
The hallmarks of fatigue in PD are extreme exhaustion and low energy levels unrelated to physical activity. It is one of the most incapacitating symptoms, decreasing the quality of life even in the early stages, and affects almost 50% of patients. In a 2015 randomized controlled trial, individuals with PD who experienced moderate-to-severe fatigue showed significantly improved scores on the Modified Fatigue Impact Scale when taking 1 mg of rasagiline daily from the beginning of treatment to the end of 12 weeks [35]. An evidence-based review of treatments for non-motor symptoms in 2019 [36], suggests that rasagiline can be an effective treatment for fatigue in patients with PD. However, the study had a small sample size, which means that the practical implications of this finding are inconclusive. Nonetheless, this finding suggests that PD patients may benefit from rasagiline to reduce fatigue. Figure 3 shows the overall treatment of PD.
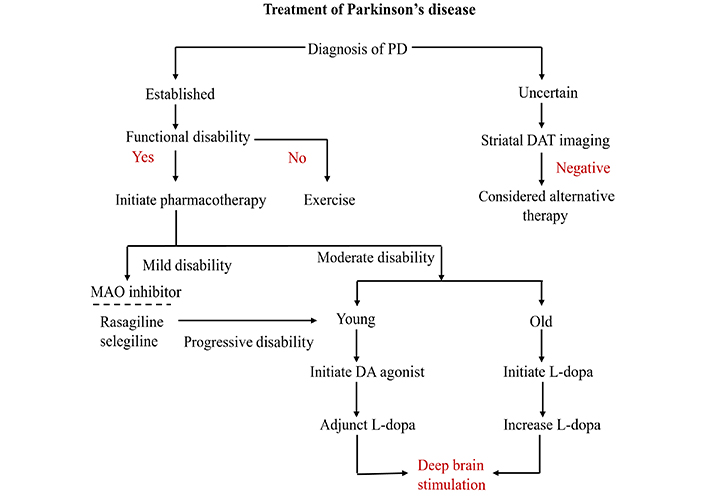
Therapeutic interventions to combat Parkinson’s disease (PD). MAO: monoamine oxidase; DA: dopamine agonist
DA is a negative modulator of gut motility. It is reported that 40–50% of body DA is produced in the gastrointestinal tract. MAO-B is an important metabolic enzyme degrading DA. Rasagiline, an irreversible MAO-B inhibitor, is used to treat PD because of its neuroprotective effect and increasing central DA. Patients with PD often experience significant GI symptoms such as gastroparesis and constipation. Constipation also is an early indicator of PD progression [37]. MAO-B inhibitors rasagiline have been reported to inhibit colonic smooth muscle contraction. Liu et al. (2018) [38] identified long-term administration of rasagiline can increase colonic DA thereby inhibiting colonic motility, suggesting that colonic MAO-B might be a potential drug target for colonic dysmotility. Rasagiline has also been reported to exert a neuroprotective effect in the CNS [38].
The plasma levels of a compound increase with higher doses. It was also observed that higher doses of selegiline led to elevated plasma selegiline levels, indicating reduced selectivity [39]. Selenium has an amphetamine skeleton in its chemical structure, which allows it to be converted into L-amphetamine and L-methamphetamine through first-pass metabolism. Selegiline has so far been regarded as a well-tolerated medication [40], despite these aspects that increase the possibility of side effects. Headaches, dizziness, insomnia, nausea, xerostomia, and constipation are among the most common adverse events reported with selegiline [41]. Furthermore, there is no difference in the frequency of adverse events between the selegiline and placebo groups, per the meta-analysis [42]. The question of whether selegiline metabolites can result in adverse effects similar to amphetamines, such as problems with the CNS and cardiovascular system, is still up for debate.
Evidence-based medicine guidelines suggest that, in early PD, DAs have more adverse effects and a higher percentage of patients discontinue treatment due to poor tolerability compared to MAO-B inhibitors. Specific side effects are rare with MAO-B inhibitors, whereas DAs are more likely to cause hallucinations [43].
A meta-analysis found no difference in deaths between the MAO-B inhibitor and control groups [44]. In addition, selegiline often causes hallucinations in patients with PD. Even with a low dose of levodopa, a high dose of selegiline is sufficient to induce hallucinations. The intensity of these hallucinations was strongly associated with disease duration. Consequently, it is prudent to advise selegiline users to limit their consumption of foods that are high in tyramine. When prescribing selegiline to patients with PD who experience postural hypotension, cardiovascular conditions, or hallucinations, caution should be exercised.
Rasagiline’s long-term safety and effectiveness as a monotherapy have been demonstrated, with no chance of a tyramine interaction. Rasagiline improved motor symptoms over 52 weeks but remained well tolerated [45]. According to a review of data from 1,504 patients with PD, when rasagiline was used along with antidepressants to treat patients with PD, the mean duration of antidepressant use was several weeks. During adjunct therapy, patients do not experience serotonin syndrome [46].
Despite several clinical trials, PD remains an incurable disease. Except for the rasagiline trial, clinical research has not provided sufficient evidence to support the idea that neuroprotection in PD can reverse the death of nigrostriatal dopaminergic neurons and prevent disease progression. The MAO-B inhibitors rasagiline and selegiline can regulate cell toxicity, AS aggregation, and mitochondrial apoptosis, all of which have strong neuroprotective effects [47]. According to animal research, Injectable rasagiline, based on a long-acting injectable formulation system, has been shown to effectively reduce motor symptoms and increase dopamine levels in rats with PD [48]. According to these studies, novel MAO-B inhibitor formulations may be crucial for patients with dysphagia, as well as for enhancing the bioavailability of the drug and patient compliance.
Third-generation MAO-B inhibitors have emerged, offering increased selectivity and potential for neuroprotection. Therefore, it is important to investigate the clinical efficacy of these inhibitors. Recent research suggests that MAO remains a favorable molecular target for studying neurodegenerative diseases. Many new, highly selective, reversible MAO-B inhibitors with minimal side effects are still being developed [49]. Scientists anticipate a new treatment approach for neurodegenerative disorders, such as Parkinson’s, as drugs that inhibit both MAO-B and cholinesterase have been developed [50]. MAO-B inhibitors have gained much attention as a potential treatment for disorders related to aging and apoptosis, in addition to PD. Randomized controlled trials have shown that patients with amyotrophic lateral sclerosis (ALS) can tolerate rasagiline. A post hoc analysis of the results suggests that rasagiline might offer protection to patients with rapidly progressing ALS [51].
MAO-B inhibitors are essential for the treatment of PD, improving dopamine signaling, and potentially slowing disease progression through various mechanisms. These inhibitors have demonstrated potential in treating motor symptoms and providing neuroprotection in both the initial and advanced stages of PD. Additionally, they have shown potential for treating non-motor symptoms, such as fatigue and sleep disturbances. While MAO-B inhibitors usually have fewer side effects than other drugs, such as DAs, caution should be exercised due to the possibility of hallucinations and tyramine interactions, particularly with selegiline. Future research and development of MAO-B inhibitors is aimed at enhancing selectivity, reversibility, and safety, which holds hope for improved treatment approaches for PD and other neurodegenerative disorders.
AS: alpha-synuclein
CNS: central nervous system
DAs: dopamine agonists
MAO-B: monoamine oxidase-B
PD: Parkinson’s disease
ROS: reactive oxygen species
UPDRS: Unified Parkinson’s Disease Rating Scale
The authors thank the Chettinad Academy of Research and Education for their constant support and encouragement.
PKCS and SMT: Writing—original draft, Writing—review & editing. RV: Conceptualization, Writing—review & editing, Supervision.
The authors declare no conflicts of interest to report.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
