Abstract
Poly-ADP-ribose polymerase inhibitors (PARP-I) represent one of the most attractive and promising class of biological agents studied both in relapsed ovarian cancer (OC) and in the advanced setting. The availability of this new class of drugs has changed the clinical management of OC ensuring an unprecedented advance in such an aggressive cancer. Three oral PARP-I are currently available: olaparib, niraparib and rucaparib. Another two are in active clinical exploration: veliparib and talazoparib. Here the authors report clinical data with PARP-I with a particular emphasis on the phase II and III trials that support PARP-I approval by regulatory agencies in OC patients.
Keywords
PARP-inhibitors, ovarian cancer, BRCA1 and 2, homologous recombination deficiency, DNA repairPARP-I development
Mechanisms of DNA repair
Five different mechanisms support, with different degrees of fidelity, genomic stability during the cell cycle: base excision repair (BER), nucleotide excision repair (NER), mismatch repair (MMR), homologous recombination (HR) and non-homologous end joining repair (NHEJ) [1]. Poly-ADP-ribose polymerase (PARP) family proteins encompasses 17 different nuclear enzymes but only PARP-1, PARP-2 and PARP-3 have a recognized role in the DNA repair machinery with PARP 1 as the most frequent isoform. PARP acts in several DNA repair mechanisms but is most extensively described in BER where it promotes the formation of DNA repair complexes [2]. However, PARP1 is known to play a crucial role also in HR facilitating the process throughout all the phases [3].
The development of PARP inhibitors (PARP-I) stems from the observation that these molecules were able to selectively kill cancer cells defective of BRCA 1 and 2 tumour suppressors [4, 5]. This capability resides on the mechanism that thereafter is called “synthetic lethality” (Figure 1). Based on this model, the absence of either one of the DNA repair activities is not lethal for the cell. But combining the deficiency of BRCA-mediated mechanism of repair (HR) with inhibition of PARP becomes highly lethal. Furthermore, other proteins have been related to the potential increased sensitivity to PARP-I, but their role in the clinical setting is still unclear [6]. Since the observation that BRCA defective tumours derive a great benefit from PARP treatment [7], ovarian cancer (OC) has been the privileged ground of development of these agents.
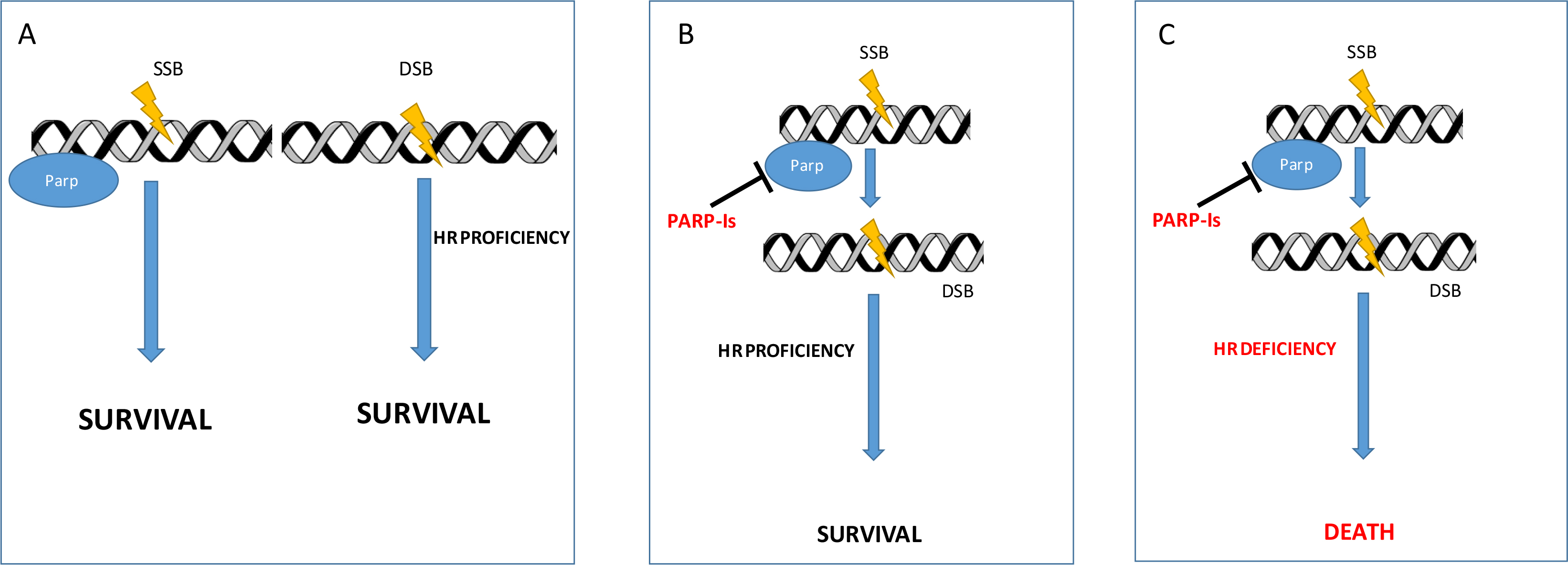
Synthetic lethality. (A) Normal cell with all the DNA repair mechanisms functioning; (B) PARP inhibition determines SSB accumulation that are effectively repaired in HR proficient cells; (C) PARP inhibition determines SSB accumulation that ultimately ends in multiple DSB cannot be repaired by HR deficient cells (synthetic lethality). DSB: double-strand break; SSB: single-strand breaks
PARP-I for recurrent disease
PARP-I efficacy and safety have been extensively investigated in relapsed OC patients. Three oral PARP-I, olaparib, niraparib and rucaparib, currently received authorization by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) both in relapsed OC as maintenance therapy after response to platinum-containing treatment and as monotherapy treatment of BRCA1/2 mutated relapsed OC. Here we report the phase II and III trials that support PARP-I approval by regulatory agencies in relapsed OC patients.
Olaparib
Initially, the olaparib authorization by the FDA and EMA was carried out with two different indications. In 2014 the FDA approved olaparib as monotherapy in germline BRCA1/2 (gBRCA) mutated advanced OC patients treated with more than three previous lines of chemotherapy (Study 42, NCT01078662). In the same period, EMA authorized olaparib (capsules formulation) as maintenance treatment in platinum-sensitive relapsed BRCA1/2 mutated high-grade serous OC (HGSOC) (Study 19, NCT00753545). Subsequently, both the FDA and EMA extended approved olaparib indication (tablet formulation) in the maintenance setting regardless of BRCA1/2 mutation (SOLO2 trial, NCT01874353).
Study 42 is a large single-arm, phase II study that evaluated olaparib capsule treatment (400 mg BID) in terms of response rate in BRCA1/2-mutated recurrent cancers (ovarian, breast, prostate, and pancreatic).
One hundred and ninety-three OC patients, heavily pre-treated, (4.3 median number of chemotherapy lines for advanced disease) were included [8]. The objective response rate was 34%. Recently, data from a subgroup of BRCA1/2 mutated OC patients (more than 3 prior chemotherapy regimens) showed a 34% overall response rate and a median response duration of 7.9 months [9].
Subsequently, the phase III trial SOLO3 (NCT02282020), enrolled 266 gBRCA mutated platinum sensitive relapsed OC patients to olaparib tablets (300 mg BID) or standard treatment (weekly paclitaxel, weekly topotecan, gemcitabine or pegylated liposomal doxorubicin). The trial confirmed olaparib efficacy and safety profile with an overall response rate (ORR) of 72% vs. 51% with chemotherapy (OR 2.53, 95% CI 1.40–4.58, P = 0.002). The most frequent adverse events (AEs) reported during olaparib treatment were nausea (65%) and anemia (50%). Regarding AEs grade ≥ 3 experienced, anemia (21% olaparib vs. 0% chemotherapy), palmar-plantar erythrodysesthesia (0% olaparib vs. 12% chemotherapy) and neutropenia (6% vs. 11%) were reported [10].
In relapsed platinum sensitive OC maintenance setting, olaparib has been evaluated in two international prospective (Study 19 and SOLO2/ENGOT-Ov21) trials [11].
Study 19 is a randomized phase II trial. Two hundred sixty-five patients with relapsed high grade serous OC, in complete (CR) or partial response (PR) to platinum-containing chemotherapy were treated with olaparib (n = 136) or placebo (n = 129) 400 mg (capsules formulation) twice daily. Olaparib treatment shows a significantly longer median progression-free survival (PFS) compared with placebo (8.4 vs. 4.8 months, HR 0.35, P < 0.001) [11]. In the BRCA1/2 (somatic and germline) mutated patients the median PFS was 11.2 vs. 4.3 months for placebo (HR 0.18, P < 0.001), as shown in a study 19 pre-planned subgroup analysis (136 patients) [12].
No significant difference in terms of overall survival (OS) was observed in overall population and in BRCA mutated patients: intention-to-treat (ITT) population OS was 29.8 vs. 27.8 months; (HR 0.73, P = 0.025), in BRCA1/2 mutated patients OS was 34.9 vs. 30.2 months; (HR 0.62, P = 0.025) [13]. AEs olaparib related were nausea, fatigue, vomiting, and anemia, which were mostly of grade 1 or 2 and arose within the first 4–8 weeks of treatment. No significant detrimental effects in terms of quality of life were observed.
Subsequently, SOLO2/ENGOT-Ov21 trial confirmed the Study 19 results with a new olaparib formulation (tablet vs. capsules). SOLO2/ENGOT-Ov21 is a phase III trial designed to assess efficacy and safety of olaparib 300 mg twice daily vs. placebo in BRCA1/2 mutated patients in response to platinum-containing chemotherapy. Stratification criteria were response to previous platinum chemotherapy (complete or partial) and platinum-free interval (> 6–12 months or > 12 months). Olaparib treatment showed an impressive and significant PFS when compared to placebo (19.1 vs. 5.5 months, HR 0.30, P < 0.0001).
Nausea, fatigue, vomiting, and anemia, primarily of grade 1 or 2, were the AEs more frequently reported in olaparib arm [14].
On August 2017 and May 2018, the FDA and EMA respectively extended olaparib indication as maintenance treatment of platinum sensitive relapsed OC patient, in response to last platinum-based chemotherapy, irrespective of BRCA status.
Niraparib
Niraparib is a selective inhibitor of PARP-1 and PARP-2 enzymes. The FDA and EMA niraparib approval (March and November 2017 respectively) comes from the ENGOTOV16/NOVA trial.
ENGOT OV16/NOVA trial is a phase III, double-blinded, trial that enrolled relapsed platinum sensitive OC patients treated with at least two previous platinum-based regimens.
The trial includes patients with measurable disease less than 2 cm treated within 8 weeks of last platinum chemotherapy regimen. Two hundred and three BRCA mutated and 350 non-gBRCA mutated patients assessed with Myriad BRCAnalysis test, were randomized 2:1 to niraparib 300 mg/day or placebo. The primary outcome of the study was PFS.
The PFS was 21 vs. 5.5 months (HR 0.27, P < 0.0001) in the BRCA mutated population, while in the wild type gBRCA patient PFS was 9.3 vs. 3.9 months (HR 0.45, P < 0.001). In homologous recombination deficiency (HRD)-positive cohort, according to Myriad Genetics myChoice HRD test, PFS was 12.9 vs. 3.8 months (HR 0.38, P < 0.001) [15].
Functional Assessment of Cancer Therapy-Ovarian Symptom Index reveals no significant detrimental effects [16]. We are still awaiting OS data, but the estimated analysis of PFS at 24 months in the niraparib arm was 0.42 (0.30, 0.55) vs. 0.16 (0.07, 0.28) (gBRCAmut) and 0.27 (0.19, 0.35) vs. 0.12 (0.06, 0.21) (non-gBRCAmut) [17].
Niraparib’s AE are similar to the PARP-I class. However, grade 3–4 thrombocytopenia, is reported in 34% of patients and typically appears during the first three cycles of therapy. Other grade G3-G4 AE reported to treatment are anemia (25%) and neutropenia (20%). If a dose reduction is correctly performed, only 3%, 2%, and 1% of patient will stop niraparib treatment due to thrombocytopenia, neutropenia, and anemia.
Body weight < 77 kg or basal platelet count < 150, 000 μL are risk factors to develop grade 3 thrombocytopenia (exploratory analysis): in this setting of patient the start dose of niraparib at 200 mg/day when one or both risk factors are present did not impact on PFS [18].
Regarding monotherapy treatment, the single arm Quadra trial (NCT02354586) results are available [19]. The study is a single arm trial evaluating niraparib efficacy in 45 platinum sensitive, HRD-positive relapsed OC patients treated with or more than three previous chemotherapy lines and PARP-I treatment naive. The trial shows an ORR of 27.5% and a disease control rate of 68.6% with duration of response of 9.2 months [19]. Quadra trial supports the recent (October 2019) FDA approval for niraparib in advanced HRD-positive OC patients treated with more than 3 previous chemotherapy regimens.
Rucaparib
Rucaparib is a potent PARP-1, 2 and 3 inhibitor approved by FDA and EMA for the treatment of patients with BRCA1/2 mutated (germline or somatic) OC treated with two or more lines of previous chemotherapy [20].
Data about rucaparib efficacy for this setting are drawn from Study 10 and ARIEL2 (NCT01891344) clinical trials. [21, 22].
Study 10 is a three-part phase I/II clinical trial: part 1 determined as 600 mg twice daily the maximum tolerable dose (MTD) and assessed the drug pharmacokinetics. In the phase II, 42 platinum sensitive (platinum free interval more than 6 months) patients with high-grade OC already treated with two to four previous regimens were enrolled. The investigator-assessed ORR was 59.5%, with a median duration of response of 7.8 months (95% CI 5.6–10.5) [21].
According to encouraging results, ARIEL2 trial was conducted [22].
The ARIEL2 study is a multicenter, phase II open label trial evaluating the activity of rucaparib as single agent in relapsed HGSOC or high-grade endometrioid ovarian carcinoma (HGEOC) regardless BRCA mutation status in patients treated with at least one prior platinum containing regimen. Two hundred and four patients (n = 40 BRCA mutated, 82 BRCA wild type/LOH high, n = 70 BRCA wild type/LOH low, n = 12 wild type/LOH unclassified) were enrolled.
According to HRD, patients were classified into three subgroups: BRCA mutated (pathogenetic germline or somatic), BRCA wild-type and LOH high group, and BRCA wild-type and LOH low group. The biomarker chosen for HRD was genomic LOH, with a cutoff to define LOH high of ≥ 14%.
The study reported a median PFS of 12.8 months (95% CI 9.0–14.7) for BRCA mutated group, in contrast to 5.7 months (95% CI 5.3–7.6) and 5.2 months (95% CI 3.6–5.5) in the LOH high and low groups, respectively. This study allowed the optimization of LOH degree associated with clinical efficacy that was prospectively defined for ARIEL3 phase III trial and Part 2 of ARIEL2 as ≥ 16%.
Subsequently, a pooled analysis from 106 patients (n = 42 from Study 10 Part 2A, n = 64 from ARIEL2 Parts 1 and 2) BRCA mutated high-grade relapsed OC after two or more chemotherapy lines treated with rucaparib at a starting dose of 600 mg twice daily was performed. Patients were already treated with a median of three prior lines of chemotherapy (range, 2–6), and 43% of patients had received at least three prior platinum-containing therapies. The ORR was 53.8% (95% CI 43.8–63.5) with 8.5% and 45.3% of patients in CR and PR, respectively. The median duration of response was 9.2 months (95% CI 6.6–11.6).
Grade ≥ 3 anemia was reported in 24.9% of patients. Treatment interruption, dose reduction, and treatment discontinuation related to AEs was reported in 58.6%, 45.9%, and 9.8% of patients, respectively [23].
Rucaparib received the FDA (April 2018) and EMA (January 2019) approval as maintenance treatment for recurrent epithelial OC, achieving a CR or PR to platinum-containing chemotherapy according to ARIEL3 trial (NCT01968213) results.
In this study, 564 patients previously treated with more than 2 prior lines of chemotherapy were randomized 2:1 to receive rucaparib or placebo maintenance (600 mg oral BID). Enrollment was not dependent on BRCA molecular analysis, patients were stratified into 3 groups: a BRCA-mutant group, an HRD-positive group, and an intent-to-treat group (all patients).
A median PFS of 16.6 months in the rucaparib arm and 5.4 months in the placebo arm (HR 0.23, 95% CI 0.16–0.34, P < 0.0001) for BRCA-mutated patients was reported; in the HRD-positive population PFS was 13.6 vs. 5.4 months (HR 0.32, 95% CI 0.24–0.42, P < 0.0001) while in the overall population (ITT) patients, 10.8 and 5.4 months respectively (HR 0.36, 95% CI 0.30–0.45, P < 0.0001) [24].
ARIEL3 trial confirmed the efficacy of PARP-I, regardless of BRCA mutations or the HRD status. Grade 3/4 AEs were reported in 56% for the rucaparib arm vs. 15% in the placebo arm. The most common reported AEs were anemia (19% vs. 1%) and an increased alanine or aspartate aminotransferase (10% vs. 0%). To assess the HRD status, the FDA simultaneously approved, FoundationFocus™ CDx BRCA LOH as companion diagnostic test [25].
Finally, ARIEL 4 (NCT02855944) is an ongoing phase III study evaluating the rucaparib activity (600 mg BID) vs. standard chemotherapy (weekly paclitaxel or platinum-containing regimen for platinum free interval < 12 months and > 12 months respectively) in BRCA mutated OC patients treated with more than two previous lines.
Veliparib
Veliparib is a small oral PARP-I with selective activity for PARP1 and PARP2 with a weaker trapping compared with other PARP-I. A phase I/II trial (NCT01472783) involving gBRCA1/2 mutated relapsed OC patient, established the MTD as 300 mg twice daily with an ORR of 65%.
Veliparib efficacy and tolerability as a single agent (400 mg twice daily) has been evaluated in the phase II clinical trial Gynecologic Oncology Group-0280 (NCT01540565). The study enrolled 45 relapsed BRCA1/2 mutated OC patients pretreated with no more than three prior chemotherapy regimens. The study reported a median PFS of 8.1 months (0.43–19.55 months) and median OS was 19.7 months with an acceptable toxicity profile [26].
Talazoparib
Talazoparib is another potent PARP1-2 inhibitor (PARP1 IC50: 0.57 nM), with a proven effectiveness direct to BRCA1/2 in vitro and in vivo models. The clinical experience of Talazoparib in OC is very limited.
A phase I/II trial (NCT01286987), conducted in 113 patients with advanced solid tumors reported an acceptable safety profile as single-agent (1.0 mg/day). In OC cohort an ORR of 42% (5/12 patients) [27] was observed.
Anemia (23%), thrombocytopenia (18%), and neutropenia (10%) were the most commonly grade 3–4 AEs reported.
In platinum-resistant OC, the combination of talazoparib with temozolomide or irinotecan demonstrated PRs in 4/7 (57%) [28].
A phase phase I/II trial of talazoparib in association with carboplatin in patients with solid tumors demonstrated a 52% of disease stabilization (range, 7–22 weeks; median, 10.5 weeks) [29].
Front line
In 2018, the results of the SOLO-1 trial investigating olaparib in maintenance after front-line platinum based chemotherapy were published [30]. In this trial, 391, BRCA1/2 mutated (deleterious or suspected deleterious germline or somatic mutation), FIGO III-IV, high grade OC patients were randomised (2:1) to receive either olaparib or placebo (300 mg BID or placebo for 2 years) as maintenance after partial or complete response to front-line platinum based chemotherapy. The study showed an unprecedented result in term of the primary outcome (PFS). In particular, the Kaplan-Meier estimate of the risk of being progression/death free at 3 years was 60% for patients receiving olaparib vs. 27% for those receiving placebo (HR = 0.030, 95% CI 0.23–0.41) [30]. Unfortunately, at the time of the results presentation the data on overall survival were not mature and we do not have the mature data yet. Based on these data the FDA and EMA approved olaparib as maintenance therapy for all the BRCA1/2 mutated OC patients, experiencing an objective response after a front-line platinum-based treatment.
More recently, the PAOLA-1 trial [31] tested the hypothesis that olaparib added in maintenance to bevacuizumab improves the PFS as compared with bevacizumab alone, in patients who received bevacizumab as part of the front-line treatment.
In this investigator initiated trial, were enrolled 762 patients who received at least 3 bevacizumab courses along with the platinum based chemotherapy. Although patients were allowed to participate irrespective of the BRCA status, BRCA mutation has been addressed for all the patients and HRD (Myriad MyChoice ≥ 42) for the vast majority (82%) of the total. Patients with high grade serous or endometroid OC treated with carboplatin-paclitaxel-bevacizumab chemotherapy in partial or complete response, were randomized (2:1) to continue bevacizumab alone (15 mg/kg every 3 weeks on day 1) or bevacizumab in combination with olaparib (300 mg twice daily for up to 24 months) for 24 months.
Main analysis showed a significant benefit with olaparib (22.1 vs. 16.6 months, HR = 0.59, 95% CI 0.49–0.72) in the overall population. Also, in this case the maximum benefit was observed in the BRCA mutant population (HR = 0.31, 95% CI 0.20–0.47) and in the HRD patients (HR = 0.33, 95% CI 0.25-0.45) with the median PFS reaching 37 months.
Interestingly, the safety data showed a reasonable profile for the combination, although with an increased incidence of AEs in the experimental arm: grade ≥ 3 AEs occurred in 57% vs. 51% of patients receiving olaparib/bevacizumab and placebo/bevacizumab, respectively, most commonly hypertension (19% vs. 30%) and anemia (17% vs. < 1%).
In the PRIMA study, 733 patients with either serous or endometroid OC with newly diagnosed stage III-IV high-grade serous OC were randomised to receive either niraparib or placebo (300 mg/once daily) for 36 months whether they had an objective response to first-line chemotherapy. Interestingly, the patients were allowed into the trial irrespective of the BRCA status, although they were all tested for HRD (through Myriad MychoiceHRD®) [32]. In fact, the primary endpoint of the study, the PFS, was tested hierarchically first in the HRD population and, if statistically significant into the whole population (ITT analysis). Among the HRD patients, (i.e. MyChoice ≥ 42 or BRCA mutant) niraparib yielded a median PFS of 21.9 as compared with the 10.9 months with placebo (HR = 0.43, 95% CI 0.31–0.59). The ITT analysis confirmed an improvement of PFS with niraparib (13.8 vs. 8.2 months, HR = 0.62, 95% CI 0.50–0.76). The most commonly reported grade ≥ 3 AEs were anaemia in 31% of patients, thrombocytopenia in 29%, and neutropenia in 13% of patients overall.
The VELIA trial evaluated the potential role of veliparib combined with chemotherapy and given as maintenance as compared with platinum based chemotherapy alone [33]. In this trial 1, 140 patients, with newly diagnosed stage III-IV high-grade serous OC were randomised (1:1:1) to receive platinum-based chemotherapy alone, chemotherapy plus veliparib for 6 cycles, or chemotherapy plus veliparib followed by veliparib maintenance (veliparib throughout). In this trial BRCA and HRD assessment were done for all the patients. HRD status initially was added as strata in the randomisation after the half of the patients enrolled. For the definition of the HRD status, Myriad MychoiceHRD® > 33 threshold was used. The main comparison of the trial was between the arm with veliparib throughout and the chemotherapy alone. Also in this case, the primary outcome of the trial, the PFS, was first assessed in the BRCA mutant population then in the HRD population and, finally, in the ITT population. The results showed an increase of the PFS in all the 3 populations. In particular, the median PFS was 34.7, 31.9 and 23.5 months for the veliparib throughout arm as compared with 22 (HR 0.44, 95% CI 0.28–0.68), 20.5 (HR = 0.57, 95% CI 0.43–0.76) and 17.3 (HR = 0.68, 95% CI 0.56–0.83) months for the chemotherapy arm in the BRCA mutant, HRD and ITT populations, respectively.
Interestingly, although significantly more patients experienced toxic events in the veliparib arm, dose intensities delivered were not dissimilar among the arms, demonstrating that the combination is feasible [33]. Table 1 summarizes clinical trials results for PARP-I in ovarian cancer.
Clinical trials results for PARP Inhibitors in ovarian cancer
| Study | Phase | Patients (n) | Setting | Treatment arms | Results | P-value |
|---|---|---|---|---|---|---|
| Recurrent/relapsed setting | ||||||
| STUDY 42 [8] | II | 193 | Recurrent pre-treaed advanced OC, BRCAmut | Olaparib 400 mg BID (capsules) | ORR: 34% MDR: 7.9 months | |
| STUDY 19 [11] [12] | II | 265 | Platinum-sensitive recurrent HGSOC | Olaparib 400 mg BID (capsules) Placebo | Overall population: 8.4 vs. 4.8 months | P < 0.0001 |
| BRCAmut: 11.2 vs. 4.3 months | P < 0.0001 | |||||
| SOLO 2 [14] | III | 295 | Platinum-sensitive recurrent HGSOC or HGEOC, BRCAmut | Olaparib 300 mg BID Placebo | Median PFS | |
| 19.1 vs. 5.5 months | P < 0.0001 | |||||
| SOLO 3 [10] | III | 266 | Platinum-sensitive recurrent HGSOC BRCAmut | Olaparib 300 mg BID Single-agent nonplatinum chemotherapy (weekly paclitaxel, weekly topotecan, gemcitabine or pegylated liposomal doxorubicin) | ORR: 72.2% vs. 51.4 | P = 0.002 |
| NOVA [15] | III | 553 | Platinum-sensitive recurrent HGSOC | Niraparib 300 mg Placebo | Median PFS | |
| gBRCAmut: 21 vs. 5.5 months | P < 0.001 | |||||
| BRCAwt HRD+: 12.9 vs. 3.8 months | P < 0.001 | |||||
| Overall non-gBRCA: 9.3 vs. 3.9 months | P < 0.001 | |||||
| QUADRA [19] | II | 45 | Platinum sensitive HRD positive HGSOC | Niraparib 300 mg | ORR: 27.5% DCR: 68.6% DoR: 9.2 months | |
| STUDY 10 [21] | I/II | 42 | Platinum-sensitive recurrent HGSOC or HGEOC, gBRCAmut (phase II PART 2A) | Rucaparib 600 mg BID | ORR: 59.5% MDR: 7.8 months | |
| ARIEL 2 PART 1 [22] | II | 192 | Platinum sensitive recurrent HGSOC or HGEOC | Rucaparib 600 mg BID | Median PFS | |
| BRCAmut: 12.8 months | P < 0.0001 | |||||
| BRCAwt LOH High: 5.7 months | P = 0.011 | |||||
| BRCAwt LOH low: 5.2 months | P = 0.011 | |||||
| ARIEL 3 [24] | III | 564 | Platinum-sensitive recurrent HGSOC or HGEOC | Rucaparib 600 MG BID Placebo | Median PFS | |
| BRCAmut: 16.6 vs. 5.4 months | P < 0.0001 | |||||
| HRD+: 13.6 vs. 5.4 months | P < 0.0001 | |||||
| ITTP: 10.8 vs. 5.4 months | P < 0.0001 | |||||
| Front-line | ||||||
| SOLO1 [30] | III | 391 | FIGO Stage III/IV HGSOC or HGEOC BRCAmut | Olaparib 300 mg BID Placebo | BRCAmut: > 36 (NR) vs. 13.8 months | P < 0.0001 HR: 0.30 (95% CI 0.23–0.41) |
| PAOLA-1 [31] | III | 806 | FIGO Stage III/IV HGSOC or HGEOC | Bevacizumab: 15 mg/kg, q21 × 15 months, including when administered with chemotherapy + olaparib (300 mg BID) × 24 months Bevacizumab: 15 mg/kg, q21 × 15 months, including when administered with chemotherapy + Placebo × 24 months | Overall population ITT: 22.1 vs. 16.6 months | P < 0.0001 HR: 0.59 (95% CI 0.49–0.72) |
| BRCAmut: 37.2 vs. 21.7 months | HR: 0.31 (95% CI 0.20–0.47) | |||||
| HRD (including BRCA): 37.2 months vs. 17.7 months | HR: 0.33 (95% CI 0.25–0.45) | |||||
| HRD (BRCAwt): 28.1 months vs. 16.6 months | HR: 0.43 (95% CI 0.28–0.66) | |||||
| HRP (BRCAwt): 16.9 months vs. 16.0 | HR: 0.92 (95% CI 0.72–1.17) | |||||
| PRIMA [32] | III | 733 | FIGO Stage III with residual tumor IV HGSOC or HGEOC | Niraparib 300 mg once daily, 36 months Placebo once daily, 36 months | Overall population: 13.8 vs. 8.2 months | P < 0.001 HR: 0.62 (95% CI 0.50–0.76) |
| BRCAmut: 22.1 vs. 10.9 months | HR: 0.40 (95% CI 0.27–.62) | |||||
| HRD (including BRCA): 21.9 vs. 10.4 months | HR: 0.43 (95% CI 0.31–0.59) | |||||
| HRD (BRCAwt): 19.6 vs. 8.2 months | HR: 0.50 (95% CI 0.31–0.83) | |||||
| HRP (BRCAwt): 8,1 vs. 5.4 months | HR: 0.68 (95% CI 0.49–0.94) | |||||
| VELIA [33] | III | 1140 | FIGO Stage III/IV HGSOC | Veliparib 150 BID in combination, 400 mg BID in maintenance | Overall population: 23.5 vs. 17.3 months | P < 0.001 HR: 0.68 (95% CI 0.56–0.83) |
| BRCAmut: 34.7 vs. 22.0 months | HR: 0.44 (95% CI 0.28–0.68) | |||||
| HRD (including BRCA): 31.9 vs. 20.5 months | HR: 0.57 (95% CI 0.43–0.76) | |||||
| HRD (BRCAwt): 22.9 vs. 19.8 months | HR: 0.74 (95% CI 0.52–1.06) | |||||
| HRP (BRCAwt): 15.0 vs. 11.5 months | HR: 0.81 (95% CI 0.6–1.09) | |||||
NR: not reached; wt: wild type
Conclusion and future perspectives
The recent success of PARP-I in OC represents an unprecedented advance in the therapeutic research for this tumour.
Of note, the evidence that BRCA mutated patients derive a great and long-lasting benefit prompted most of the clinicians caring for OC patients to actively search for this mutation occurring in almost 20% of the patients. Since BRCA mutation is associated with a significant risk of inherited breast and ovarian cancer, this active search highlighted and in some cases improved the way these patients and mostly their families are cared. The recent data showing some activity also in patients who are proficient in HR or BRCA wild type threaten this possibility as the regulatory approval allows to treat all the patients with PARP-I.
Generally, the questions regarding who should be treated and when the PARP-I should be integrated into the OC patients treatment are the two most important issues the clinicians face in deciding the best strategy for these patients.
The answer to these questions is not easy, since none of these trials reports a mature OS analysis that could ultimately help in deciding the best treatment. Moreover, in most of the trials the control arm is represented by chemotherapy alone, whilst the current standard of care is chemotherapy plus bevacizumab (given in combination and as maintenance) and also in the recurrent setting trials most of the patients enrolled were not pretreated with bevacizumab. The only trial in which maintenance with olaparib plus bevacizumab was compared with bevacizumab maintenance alone is PAOLA-1. By design this trial answers to the question of whether maintenance bevacizumab plus olaparib is better than bevacizumab alone. And the data suggest this conclusion. However we lack some important pieces of information on whether PARP-I alone is better than bevacizumab alone (in maintenance) in patients who received bevacizumab in combination with the induction chemotherapy or, based on SOLO-1, at least BRCA mutant could derive greater or similar benefit from olaparib alone maintenance instead of the combination olaparib plus bevacizumab, sparing some important toxicity. All these questions deem to be answered in a prospective randomised clinical trial, in our opinion.
Finally, since PARP-I are both approved in recurrent disease and will be approved with various labels in front-line two other questions are arising from the data published up-to-date.
When to administer PARP-I? Front-line or at the recurrence? Is there any role for PARP-I after progression or recurrence while on PARP-I?
The first question is threatened by the lack of OS data for the settings. Also in this case a meaningfully designed clinical trial could answer to this question. The second issue is being explored by the OREO study that is still recruiting (NCT03106987). In this trial, OC patients with the disease relapsing/progressing while on PARP-I and experiencing a complete or partial radiological response to subsequent treatment with platinum-based chemotherapy are randomised to receive olaparib or placebo (2:1).
In conclusion, the advent of PARP-I in OC represents an important resource in the clinical setting for the treatment of patients whose therapy did not greatly change over 20 years and mostly, is still an exciting challenge for the research in this field.
Abbreviations
| AE: | adverse event |
| BER: | base excision repair |
| CR: | complete response |
| EMA: | European Medicines Agency |
| FDA: | Food and Drug Administration |
| gBRCA: | germline-BRCA |
| HGEOC: | high-grade endometrioid ovarian cancer |
| HGSOC: | high-grade serous ovarian cancer |
| HR: | homologous recombination |
| HRD: | homologous recombination deficiency |
| ITT: | intention-to-treat |
| LOH: | loss of heterozygosity |
| MMR: | mismatch repair |
| NER: | nucleotide excision repair |
| NHEJ: | non-homologous end joining |
| OC: | ovarian cancer |
| ORR: | overall response rate |
| OS: | overall survival |
| PARP: | Poly-ADP-ribose polymerase |
| PARP-I: | PARP inhibitors |
| PFS: | progression-free survival |
| PR: | partial response |
| DSB: | double-strand break |
| SSB: | single-strand breaks |
| wt: | wild type |
Declarations
Author contributions
AP wrote the majority of the first draft of the manuscript; FC, CN, GS, DL and GD wrote sections of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflicts of interest
AP declared personal fees from AstraZeneca; GD declared advisory board from BeiGene and travel from Roche.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Not applicable.
Copyright
© The Author(s) 2020.